Calicheamicin is a natural product with antitumour properties discovered in the 1980s, with the structure shown below. As noted elsewhere, this structure has many weird properties, including amongst other features an unusual “enedidyne” motif and the presence of an iodo group on an aromatic ring. Its isolated 3D structure is quite difficult to get hold of (embedded structures in a DNA fragment are available however); the 3D model associated with the Wikipedia entry is essentially only in 2D. The representation shown below, including the absolute stereochemistry, was obtained from the SciFinder entry.
Mechanism of the Masamune-Bergman reaction. Part 2: a possible 3D Model for Calicheamicin revealing the non-covalent-interactions (NCI) present.
August 26th, 2024Mechanism of the Masamune-Bergman reaction. Part 1.
August 24th, 2024The Masamune-Bergman reaction[1],[2] is an example of a highly unusual class of chemical mechanism[3] involving the presumed formation of the biradical species shown as Int1 below by cyclisation of a cycloenediyne reactant. Such a species is so reactive that it will be quickly trapped, as for example by dihydrobenzene to form the final product. This cycloenediyne is not just an obscure chemical curiosity, the motif is incorporated into the natural product Calicheamicin, which is a potent antitumor antibiotic discovered in the 1980s. This drug owes its activity to the cyclisation TS1 shown below, which for n=2 occurs at the low temperature of 310K. The resulting biradical Int1 is a potent hydrogen abstractor, the species acting this way for hydrogen atoms associated with deoxyribose of DNA, ultimately leading to strand scission. Although I have explored many a mechanism on this blog using computational methods, I have never included any biradical examples. Here I explore the computational aspects of this reaction, and also include a pathway proceeding vis TS2- Int2 – TS3 in which hydrogen abstraction precedes cyclisation, in order to see how competitive such an alternative might be as a function of the ring size (n in scheme below).
References
- N. Darby, C.U. Kim, J.A. Salaün, K.W. Shelton, S. Takada, and S. Masamune, "Concerning the 1,5-didehydro[10]annulene system", J. Chem. Soc. D, vol. 0, pp. 1516-1517, 1971. https://doi.org/10.1039/c29710001516
- R.R. Jones, and R.G. Bergman, "p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure", Journal of the American Chemical Society, vol. 94, pp. 660-661, 1972. https://doi.org/10.1021/ja00757a071
- R.K. Mohamed, P.W. Peterson, and I.V. Alabugin, "Concerted Reactions That Produce Diradicals and Zwitterions: Electronic, Steric, Conformational, and Kinetic Control of Cycloaromatization Processes", Chemical Reviews, vol. 113, pp. 7089-7129, 2013. https://doi.org/10.1021/cr4000682
Revisiting open/transparent peer review.
July 31st, 2024Back in 2017, I was asked to peer review an article and its author asked if I would like the review to be “open” – that is that my name would be shown as a reviewer; [1]10.1073/pnas.1709586114[/cite/] indeed it was!
References
How should data be cited in journal articles? A Crossref request for public comment!
July 18th, 2024Metadata is something that goes on behind the scenes and is rarely of concern to either author or readers of scientific articles. Here I tell a story where it has rather greater exposure. For journals in science and chemistry, each article published has a corresponding metadata record, associated with the persistent identifier of the article and known to most as its DOI. The metadata contains information about the article such as its authors and their affiliations, the title of the article and its abstract, and is submitted to/registered with Crossref – an organisation set up in 1999 on behalf of publishers, libraries, research institutions and funders. Relatively recent additions to Crossref metadata are the citations included in the article, so-called Open Citations. Doing so has helped to create the new area of article metrics, used by e.g. Altmetrics or Dimensions to help identify the impacts that science publications have. Basically, if one article is cited by another, it is making an impact. Many citations of a given article by other articles means a larger impact. Most researchers love to have a high – and of course positive – impact and perhaps for better or worse, academic careers to some extent depend on such impacts.
A peak behind the (hosting) scenes of this blog.
June 15th, 2024I should start by saying that the server on which this blog is posted was set up in June 1993. Although the physical object has been replaced a few times, and had been “virtualised” about 15 years ago, a small number of the underlying software base components may well date way back, perhaps even to 1993. This system had begun to get unreliable in recent years, and it was decided about 6 months ago to build an entirely new virtual server and then migrate stuff to it.
The 100th Anniversary year of Curly Arrows.
June 14th, 2024Chemists now use the term “curly arrows” as a language to describe the electronic rearrangements that occur when a (predominately organic) molecule transforms to another – the so called chemical reaction. It is also used to infer, via valence bond or resonance theory, what the mechanistic implications of that reaction are. It was in this latter context that the very first such usage occured in 1924[1] taking the form of a letter by Robert Robinson to the secretary of the Chemical Society and “read” on December 18th 1924. The following diagram was included:
References
- "Forthcoming events", Journal of the Society of Chemical Industry, vol. 43, pp. 1295-1298, 1924. https://doi.org/10.1002/jctb.5000435208
Data Discoverability as a feature of Journal Articles.
June 11th, 2024I can remember a time when journal articles carried selected data within their body as e.g. Tables, Figures or Experimental procedures, with the rest consigned to a box of paper deposited (for UK journals) at the British library. Then came ESI or electronic supporting information. Most recently, many journals are now including what is called a “Data availability” statement at the end of an article, which often just cites the ESI, but can increasingly point to so-called FAIR data. The latter is especially important in the new AI-age (“FAIR is AI-Ready”). One attribute of FAIR data is that it can be associated with a DOI in addition to that assigned to the article itself, and we have been promoting the inclusion of that Data DOI in the citation list of the article.[1] Since the data can also cite the article, a bidirectional link between data and article is established. ESI itself can exceed 1000 “pages” of a PDF document and examples of chemical FAIR data exceeding 62 Gbytes[2] (Also see DOI: 10.14469/hpc/10386) are known. Finding the chemical needle in that data haystack can become a serious problem. So here I illustrate a recent suggestion for moving to the next stage, namely the inclusion of a “Data Availability and Discovery” statement. The below is the text of such a statement in a recently published article.[3]
References
- H. Rzepa, "The journey from Journal "ESI" to FAIR data objects: An eighteen year old (continuing) experiment.", 2023. https://doi.org/10.59350/g2p77-78m14
- T. Mies, A.J.P. White, H.S. Rzepa, L. Barluzzi, M. Devgan, R.A. Layfield, and A.G.M. Barrett, "Syntheses and Characterization of Main Group, Transition Metal, Lanthanide, and Actinide Complexes of Bidentate Acylpyrazolone Ligands", Inorganic Chemistry, vol. 62, pp. 13253-13276, 2023. https://doi.org/10.1021/acs.inorgchem.3c01506
- D.C. Braddock, S. Lee, and H.S. Rzepa, "Modelling kinetic isotope effects for Swern oxidation using DFT-based transition state theory", Digital Discovery, vol. 3, pp. 1496-1508, 2024. https://doi.org/10.1039/d3dd00246b
Possible Formation of an Impossible Molecule?
May 20th, 2024In the previous post, I explored the so-called “impossible” molecule methanetriol. It is regarded as such because the equilbrium resulting in loss of water is very facile, being exoenergic by ~14 kcal/mol in free energy. Here I explore whether changing the substituent R could result in suppressing the loss of water and stabilising the triol.
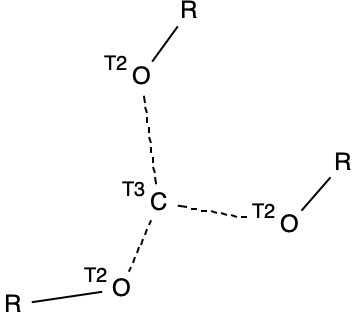
I started (as I usually do) with a search for crystal structures, in this case containing the motif shown below (trisubstituted carbon, disubstituted oxygen and R = H or C and any type of connecting bond), which is the species resulting from loss of R– to form a trihydroxycarbenium cation.
Exploring Methanetriol – “the Formation of an Impossible Molecule”
May 16th, 2024What constitutes an “impossible molecule”? Well, here are two, the first being the topic of a recent article[1]. The second is a favourite of organic chemistry tutors, to see if their students recognise it as an unusual (= impossible) form of a much better known molecule.
References
- J.H. Marks, X. Bai, A.A. Nikolayev, Q. Gong, C. Zhu, N.F. Kleimeier, A.M. Turner, S.K. Singh, J. Wang, J. Yang, Y. Pan, T. Yang, A.M. Mebel, and R.I. Kaiser, "Methanetriol─Formation of an Impossible Molecule", Journal of the American Chemical Society, vol. 146, pp. 12174-12184, 2024. https://doi.org/10.1021/jacs.4c02637
Detecting anomeric effects in tetrahedral boron bearing four oxygen substituents.
April 30th, 2024In an earlier post, I discussed[1] a phenomenon known as the “anomeric effect” exhibited by tetrahedral carbon compounds with four C-O bonds. Each oxygen itself bears two bonds and has two lone pairs, and either of these can align with one of three other C-O bonds to generate an anomeric effect. Here I change the central carbon to a boron to explore what happens, as indeed I promised earlier.
References
- H. Rzepa, "Detecting anomeric effects in tetrahedral carbon bearing four oxygen substituents.", 2024. https://doi.org/10.59350/dfkt5-k2b20