A word of explanation about this test page for experimenting with JSmol. Many moons ago I posted about how to include a generated 3D molecular model in a blog post, and have used that method on many posts here ever since. It relied on Java as the underlying software (first introduced in 1996), or almost 20 years ago. Like most software technologies, much has changed, and Java itself (as a compiled language) has had to move to improve its underlying security. In the last year, the Java code itself (in this case Jmol) has needed to be digitally signed in a standard manner, and this meant that many an old site that used unsigned older versions has started to throw up increasingly alarming messages.
Test of JSmol in WordPress: the background story.
June 8th, 2014Kekulé’s vibration: A modern example of its use.
June 6th, 2014Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane. The idea was sparked by Steve Bachrach’s latest post, where the “zero-point” structure of the molecule has recently been clarified as having D2 symmetry.[cite]10.1002/chem.201304972[/cite]
Benzene: an oscillation or a vibration?
May 28th, 2014In the preceding post, a nice discussion broke out about Kekulé’s 1872 model for benzene.[cite]10.1002/jlac.18721620110[/cite] This model has become known as the oscillation hypothesis between two extreme forms of benzene (below). The discussion centered around the semantics of the term oscillation compared to vibration (a synonym or not?) and the timescale implied by each word. The original article is in german, but more significantly, obtainable only with difficulty. Thus I cannot access[cite]10.1002/jlac.18721620110[/cite] the article directly since my university does not have the appropriate “back-number” subscription.‡ So it was with delight that I tracked down an English translation in a journal that I could easily access.[cite]10.1039/JS8722500605[/cite] Here I discuss what I found (on pages 614-615, the translation does not have its own DOI).
An original chemistry lab from the early 19th century.
May 18th, 2014Not a computer in sight! I refer to a chemistry lab from the 1800s I was recently taken to, where famous french chemists such as Joseph Gay-Lussac, Michel Chevreul and Edmond Fremy were professors. Although not used for chemistry any more, it is an incredible treasure trove of objects. Here are photos of some.
A newcomer in the game of how we find and use data.
May 17th, 2014I remember a time when tracking down a particular property of a specified molecule was an all day effort, spent in the central library (or further afield). Then came the likes of STN Online (~1980) and later Beilstein. But only if your institution had a subscription. Let me then cut to the chase: consider this URL: http://search.datacite.org/ui?q=InChIKey%3DLQPOSWKBQVCBKS-PGMHMLKASA-N The site is datacite, which collects metadata about cited data! Most of that data is open in the sense that it can be retrieved without a subscription (but see here that it is not always made easy to do so). So, the above is a search for cited data which contains the InChIkey LQPOSWKBQVCBKS-PGMHMLKASA-N. This produces the result:
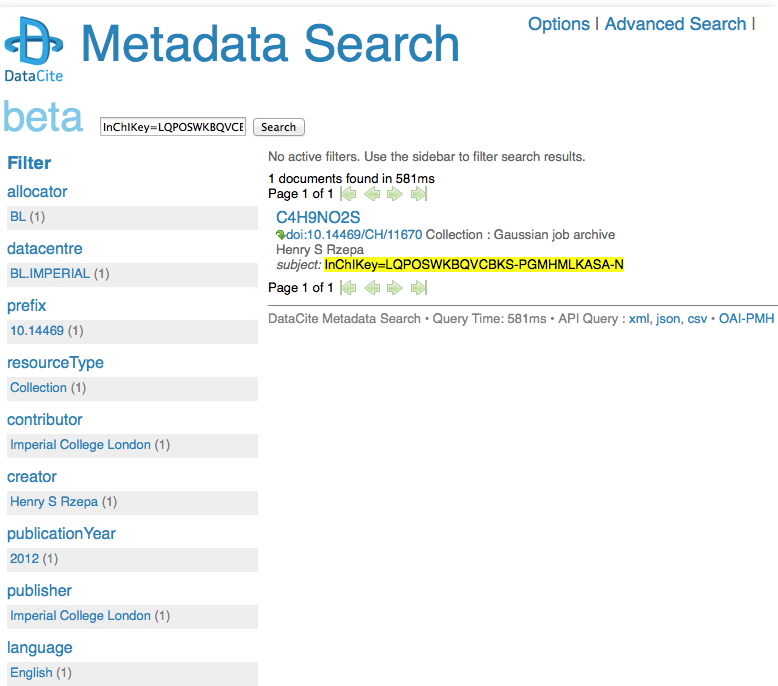
This tells you who published the data (but oddly, its date is merely to the nearest year? It is beta software after all). The advanced equivalent of this search looks like this:
Disambiguation/provenance of claimed scientific opinion and research.
May 5th, 2014My name is displayed pretty prominently on this blog, but it is not always easy to find out who the real person is behind many a blog. In science, I am troubled by such anonymity. Well, a new era is about to hit us. When you come across an Internet resource, or an opinion/review of some scientific topic, I argue here that you should immediately ask: “what is its provenance?”
Trigonal bipyramidal or square pyramidal: Another ten minute exploration.
May 2nd, 2014This is rather cranking the handle, but taking my previous post and altering the search definition of the crystal structure database from 4- to 5-coordinate metals, one gets the following.
Ribulose-1,5-bisphosphate + carbon dioxide → carbon fixation!
April 20th, 2014Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.