Text books often show the following diagram, famously consolidated over many years by Emil Fischer from 1891 onwards. At the top sits D-(+)-glyceraldehyde, to which all the monosaccharides below are connected by painstaking chemical transformations.
Prediction preceding experiment in chemistry – how unlucky was John Kirkwood?
November 30th, 2019Some areas of science progressed via very famous predictions that were subsequently verified by experiments. Think of Einstein and gravitational waves or of Dirac and the positron. There are fewer well-known examples in chemistry; perhaps Watson and Crick’s prediction of the structure of DNA, albeit based on the interpretation of an existing experimental result. Here I take a look at a what if, that of John Kirkwood’s prediction of the absolute configuration of a small molecule based entirely on matching up the sign of a measured optical rotation with that predicted by (his) theory.
The Structure of Tetrodotoxin as a free base – with a better solvation model.
November 26th, 2019In the previous post, I discussed the structure of the free base form of tetrodotoxin, often represented as originally suggested by Woodward[cite]10.1351/pac196409010049[/cite] below in an ionic form:
The Structure of Tetrodotoxin as a free base.
November 9th, 2019The notorious neurotoxin Tetrodotoxin is often chemically represented as a zwitterion, shown below as 1. This idea seems to originate from a famous article written in 1964 by the legendary organic chemist, Robert Burns Woodward.[cite]10.1351/pac196409010049[/cite] This structure has propagated on to Wikipedia and is found in many other sources.
With the elegance and the unique style that is typical Woodward, his article is a tour de force because of the way in which he deploys a large armoury of spectroscopic (X-ray crystal,† NMR, IR) as well as physicochemical (pKa) tools to infer this structure; an approach that has been subsequently widely emulated. The article a well worth a read for the elegant logic that slowly builds to a climax on page 73 (sic!) of the article, when he unveils his final structure (XXXVIII, or 38). The lecture(s) from which the article is apparently derived must have been one hell of an occasion.‡
Does Kekulene have Kekulé vibrational modes? Yes!
October 19th, 2019Increasingly, individual small molecules are having their structures imaged using STM, including cyclo[18]carbon that I recently discussed. The latest one receiving such treatment is Kekulene.[cite]10.1021/jacs.9b07926[/cite]
Catalytic Mitsunobu reaction.
October 9th, 2019If, as a synthetic chemist, you want to invert the configuration of an alcohol in which the OH group is at a chiral centre, then the Mitsunobu reaction has been a stalwart for many years. Now a catalytic version has been published, [cite]10.1126/science.aax3353[/cite] along with a proposed mechanism. Here I apply computation as a reality check to see what the energetics of this mechanism might be.
Bond length alternation (BLA) in large conjugated rings: an (anti-aromatic) update.
October 3rd, 2019In the previous post, I looked at a class of molecule known as hexaphyrins, inspecting bond length alternation (BLA) at the so-called meso position, the carbon atom joining two pyrrole rings. A search of the difference in bond lengths at this position had shown two significant clusters of crystal structures.
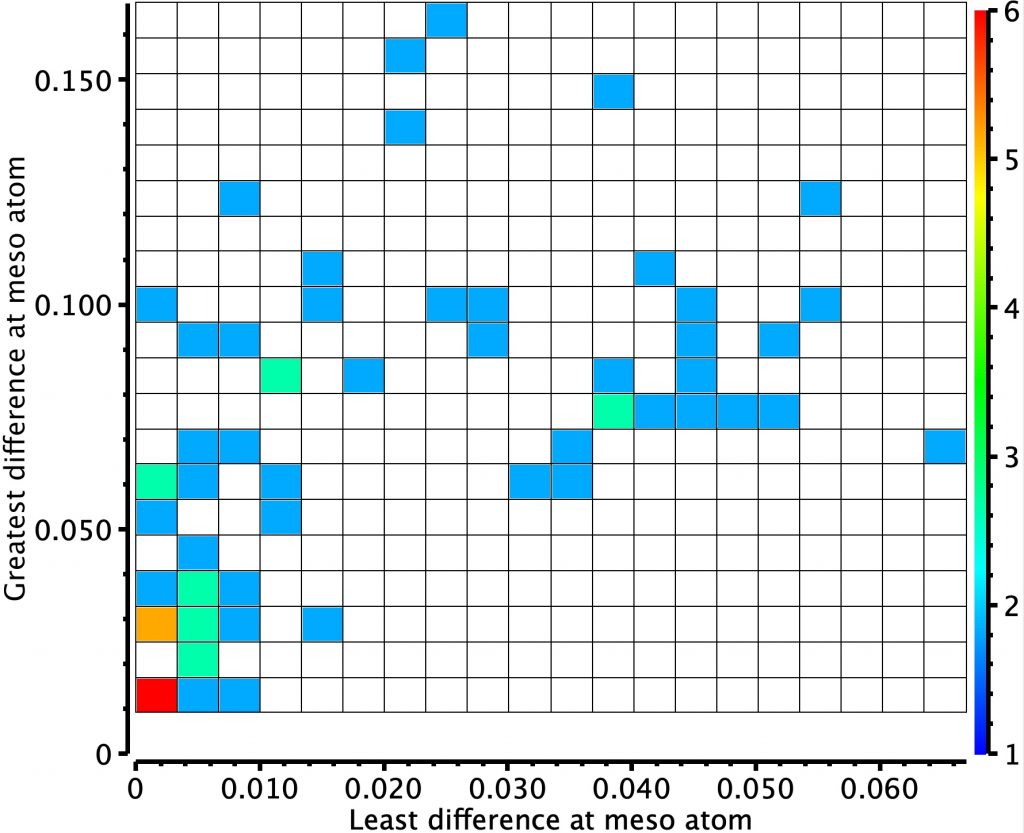 Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Bond length alternation (BLA) in large aromatic rings: an experimental reality check.
September 30th, 2019The theme of the last three posts derives from the recently reported claimed experimental observation of bond length alternation (BLA) in cyclo[18]carbon, a ring of just 18 carbon atoms.[cite]10.1126/science.aay1914[/cite] Having found that different forms of quantum calculation seem to find this property particularly difficult to agree upon, not only for cyclocarbon but for twisted lemniscular annulenes (which contain CH rather than just C units), I thought it might be time to look at some more experimental data and my chosen system is a class called the hexaphyrins, of which there are a number of experimental crystal structures.
The Kekulé vibration as a function of aromatic ring size. A different perspective using lemniscular rings.
September 27th, 2019In the previous posts, I tried to track down the onset of bond length alternation (BLA) as a function of ring size in aromatic cyclocarbons, finding the answer varied dramatically depending on the type of method used to calculate it. So here I change the system to an unusual kind of aromatic ring, the leminiscular or figure-eight annulene series.♥ I explore the Kekulé vibration for such species for which a 4n+2 π electron count means they are cyclically Möbius aromatic.[cite]10.1016/j.comptc.2014.09.028[/cite]
Cyclo[6] and [10]carbon. The Kekulé vibrations compared.
September 3rd, 2019In the previous post, I looked at the so-called Kekulé vibration of cyclo[18]carbon using various quantum methods and basis sets. Because some of these procedures can take a very long time, I could not compare them using the same high-quality consistent atom basis set for the carbon (Def2-TZVPP). Here I try to start to do this using the smaller six and ten carbon rings to see what trends might emerge. FAIR data are at DOI: 10.14469/hpc/6069