Posts Tagged ‘PDF’
Monday, March 7th, 2016
Tags:Academic publishing, chemical, chemical information division, Chemical nomenclature, chemical structures, Chemical substance, chemical/x-wavefunction, Cheminformatics, City: San Diego, content media, data repository search, format type chemical/x-* , Identifiers, Imperial College, Imperial College London, International Chemical Identifier, JSON, media types, multipurpose internet media extensions, ORCiD, PDF, potential such systems, research data management, Search queries, Technical communication, Technology/Internet
Posted in Chemical IT | 2 Comments »
Saturday, July 11th, 2015
Previously on the kinetic isotope effects for the Baeyer-Villiger reaction, I was discussing whether a realistic computed model could be constructed for the mechanism. The measured KIE or kinetic isotope effects (along with the approximate rate of the reaction) were to be our reality check. I had used ΔΔG energy differences and then HRR (harmonic rate ratios) to compute[1] the KIE, and Dan Singleton asked if I had included heavy atom tunnelling corrections in the calculation, which I had not. His group has shown these are not negligible for low-barrier reactions such as ring opening of cyclopropyl carbinyl radical.[2] As a prelude to configuring his suggested programs for computing tunnelling (GAUSSRATE and POLYRATE), it was important I learnt how to reproduce his KIE values.[2] Hence the title of this post. Now, read on.
(more…)
References
- H.S. Rzepa, "KINISOT. A basic program to calculate kinetic isotope effects using normal coordinate analysis of transition state and reactants.", 2015. https://doi.org/10.5281/zenodo.19272
- O.M. Gonzalez-James, X. Zhang, A. Datta, D.A. Hrovat, W.T. Borden, and D.A. Singleton, "Experimental Evidence for Heavy-Atom Tunneling in the Ring-Opening of Cyclopropylcarbinyl Radical from Intramolecular <sup>12</sup>C/<sup>13</sup>C Kinetic Isotope Effects", Journal of the American Chemical Society, vol. 132, pp. 12548-12549, 2010. https://doi.org/10.1021/ja1055593
Tags:activation free energy, Basis set, Dan Singleton, energy differences, gas phase calculation, Kinetic isotope effect, PDF, Physical organic chemistry
Posted in reaction mechanism | 2 Comments »
Saturday, June 20th, 2015
The university sector in the UK has quality inspections of its research outputs conducted every seven years, going by the name of REF or Research Excellence Framework. The next one is due around 2020, and already preparations are under way! Here I describe how I have interpreted one of its strictures; that all UK funded research outputs (i.e. research publications in international journals) must be made available in open unrestricted form within three months of the article being accepted for publication, or they will not be eligible for consideration in 2020.
(more…)
Tags:Academia, Academic publishing, Archival science, author, Data management, Digital library, EPrints, Institutional repository, Knowledge, Knowledge representation, Library science, metadata, Open access, PDF, personal web page, Preprint, Publishing, Repository, researcher, ROMEO GREEN, Science, Technology/Internet, United Kingdom, web server
Posted in Chemical IT | No Comments »
Thursday, January 15th, 2015
Derek Lowe in his In the Pipeline blog is famed for spotting unusual claims in the literature and subjecting them to analysis. This one is entitled Odd Structures, Subjected to Powerful Computations. He looks at this image below, and finds the structures represented there might be a mistake, based on his considerable experience of these kinds of molecules. I expect he had a gut feeling within seconds of seeing the diagram.
(more…)
Tags:created using spreadsheet software, Derek Lowe, Oxford, PDF, simulation
Posted in Chemical IT, General | No Comments »
Wednesday, November 12th, 2014
In London, one has the pleasures of attending occasional one day meetings at the Burlington House, home of the Royal Society of Chemistry. On November 5th this year, there was an excellent meeting on the topic of Challenges in Catalysis, and you can see the speakers and (some of) their slides here. One talk on the topic of Direct amide formation – the issues, the art, the industrial application by Dave Jackson caught my interest. He asked whether an amide could be formed directly from a carboxylic acid and an amine without the intervention of an explicit catalyst. The answer involved noting that the carboxylic acid was itself a catalyst in the process, and a full mechanistic exploration of this aspect can be found in an article published in collaboration with Andy Whiting's group at Durham.[1] My after-thoughts in the pub centered around the recollection that I had written some blog posts about the reaction between hydroxylamine and propanone. Might there be any similarity between the two mechanisms?
(more…)
References
- H. Charville, D.A. Jackson, G. Hodges, A. Whiting, and M.R. Wilson, "The Uncatalyzed Direct Amide Formation Reaction – Mechanism Studies and the Key Role of Carboxylic Acid H‐Bonding", European Journal of Organic Chemistry, vol. 2011, pp. 5981-5990, 2011. https://doi.org/10.1002/ejoc.201100714
Tags:Andy Whiting, Dave Jackson, dielectric, Durham, energy profile, free energy barrier, London, non-polar solution, PDF, Royal Society of Chemistry
Posted in reaction mechanism | 6 Comments »
Tuesday, September 16th, 2014
ELNs (electronic laboratory notebooks) have been around for a long time in chemistry, largely of course due to the needs of the pharmaceutical industries. We did our first extensive evaluation probably at least 15 years ago, and nowadays there are many on the commercial market, with a few more coming from opensource communities. Here I thought I would bring to your attention the potential of an interesting new entrant from the open community.
(more…)
Tags:.com, chemical sketches, Java, JavaScript, lecturer, molecular editor, PDF, pharmaceutical industries, Silicon Graphics, three technologies, web browsers, Web-based behaviour
Posted in Chemical IT | No Comments »
Saturday, July 19th, 2014
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B40 to be specific.[1] My interest was in how the σ and π-electrons were partitioned. In a C40, one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that. Having one electron less per atom, one might imagine that a fullerene-like boron cluster would have no π-electrons. But the element has a propensity[2] to promote its σ-electrons into the π-manifold, leaving a σ-hole. So how many π-electrons does B40 have? These sorts of clusters are difficult to build using regular structure editors, and so coordinates are essential. The starting point for a set of coordinates with which to compute a wavefunction was the supporting information. Here is the relevant page: 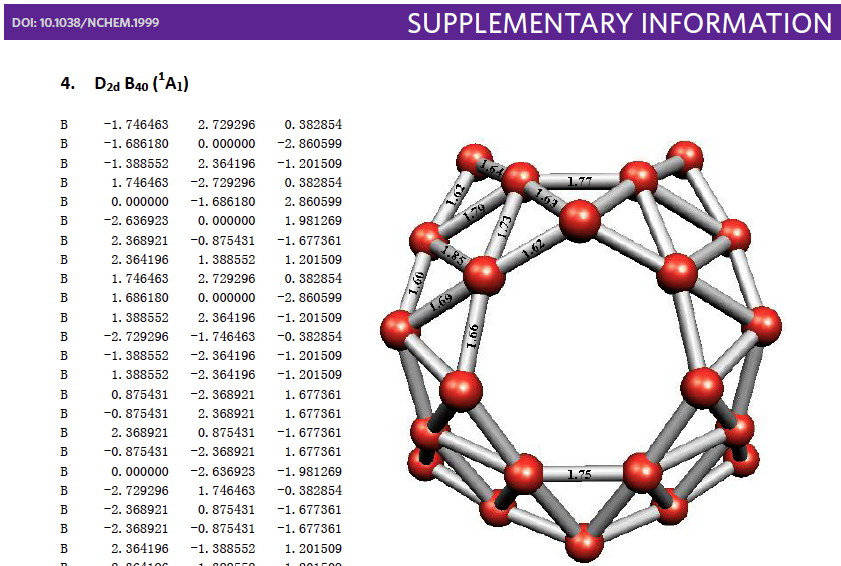 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
(more…)
References
- H. Zhai, Y. Zhao, W. Li, Q. Chen, H. Bai, H. Hu, Z.A. Piazza, W. Tian, H. Lu, Y. Wu, Y. Mu, G. Wei, Z. Liu, J. Li, S. Li, and L. Wang, "Observation of an all-boron fullerene", Nature Chemistry, vol. 6, pp. 727-731, 2014. https://doi.org/10.1038/nchem.1999
- H.S. Rzepa, "The distortivity of π-electrons in conjugated boron rings", Physical Chemistry Chemical Physics, vol. 11, pp. 10042, 2009. https://doi.org/10.1039/b911817a
Tags:Acrobat, Adobe, chemical markup, DOS, operating systems, PDF, pence, Unix
Posted in Chemical IT, Interesting chemistry | 2 Comments »
Friday, May 18th, 2012
Text books (is this a misnomer, much like “papers” are in journals?) in a higher-educational chemistry environment, I feel, are at a cross-roads. What happens next?
(more…)
Tags:author, Bob Hanson, energy, GBP, iPads, PDF, skilful author, Skolnik, Steve Job, Steve Jobs, tablet devices, textbook author, Tutorial material, USD
Posted in General | 3 Comments »
Thursday, November 10th, 2011
Fascination with nano-objects, molecules which resemble every day devices, is increasing. Thus the world’s smallest car has just been built[1]. The mechanics of such a device can often be understood in terms of chemical concepts taught to most students. So I thought I would have a go at this one!
(more…)
References
- T. Kudernac, N. Ruangsupapichat, M. Parschau, B. Maciá, N. Katsonis, S.R. Harutyunyan, K. Ernst, and B.L. Feringa, "Electrically driven directional motion of a four-wheeled molecule on a metal surface", Nature, vol. 479, pp. 208-211, 2011. https://doi.org/10.1038/nature10587
Tags:car drives, car rattling, chemical concepts, chemical name atropisomerism, chemical perspective, conformational analysis, day devices, energy, energy difference, Hubert Maehr, molecular car, nanocar, PDF, smallest car, Taxol
Posted in General, Interesting chemistry | 3 Comments »
Saturday, November 20th, 2010
For those of us who were around in 1985, an important chemical IT innovation occurred. We could acquire a computer which could be used to draw chemical structures in one application, and via a mysterious and mostly invisible entity called the clipboard, paste it into a word processor (it was called a Macintosh). Perchance even print the result on a laserprinter. Most students of the present age have no idea what we used to do before this innovation! Perhaps not in 1985, but at some stage shortly thereafter, and in effect without most people noticing, the return journey also started working, the so-called round trip. It seemed natural that a chemical structure diagram subjected to this treatment could still be chemically edited, and that it could make the round trip repeatedly. Little did we realise how fragile this round trip might be. Years later, the computer and its clipboard, the chemistry software, and the word processor had all moved on many generations (it is important to flag that three different vendors were involved, all using proprietary formats to weave their magic). And (on a Mac at least) the round-tripping no longer worked. Upon its return to (Chemdraw in this instance), it had been rendered inert, un-editable, and devoid of semantic meaning unless a human intervened. By the way, this process of data-loss is easily demonstrated even on this blog. The chemical diagrams you see here are similarly devoid of data, being merely bit-mapped JPG images. Which is why, on many of these posts, I put in the caption Click for 3D, which gives you access to the chemical data proper (in CML or other formats). And I throw in a digital repository identifier for good measure should you want a full dataset.
(more…)
Tags:Adobe, Apple, Apple iPad, ChemDraw 12, chemical data, chemical diagrams, chemical integrity, Chemical IT, chemical structure diagram, chemical structures, chemistry software, iPad, Mac, Mac OS X, Macintosh, Microsoft, opendata, PDF, Peter Murray-Rust, Postscript, word processor, XML
Posted in Chemical IT | 5 Comments »
