Symbiosis between computation and experiment is increasingly evident in pedagogic journals such as J. Chemical Education. Thus an example of original laboratory experiments[cite]10.1021/ed077p271[/cite],[cite]10.1021/ed078p1266[/cite] that later became twinned with a computational counterpart.[cite]10.1021/ed500398e[/cite] So when I spotted this recent lab experiment[cite]10.1021/acs.jchemed.7b00566[/cite] I felt another twinning approaching.
Posts Tagged ‘relative energy’
Organocatalytic cyclopropanation of an enal: (computational) mechanistic understanding.
Saturday, August 25th, 2018I’ve started so I’ll finish. The mechanism of diazo coupling to indoles – forty (three) years on!
Thursday, December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then. In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[cite]10.1039/P29750001209[/cite],[cite]10.5281/zenodo.18777[/cite] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
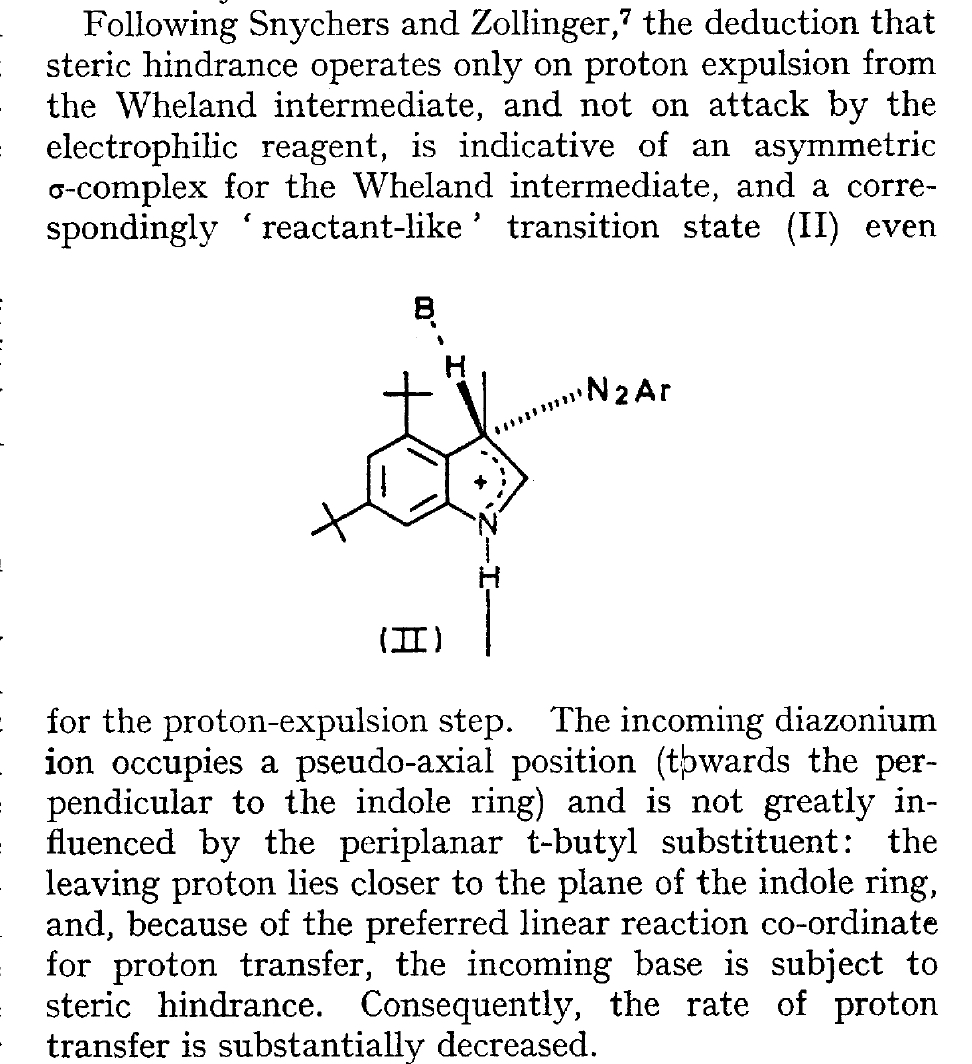 The main points of this argument were;
The main points of this argument were;
Modelling the geometry of unbranched alkanes.
Saturday, March 29th, 2014By about C17H36, the geometry of “cold-isolated” unbranched saturated alkenes is supposed not to contain any fully anti-periplanar conformations. [cite]10.1002/anie.201202894[/cite] Indeed, a (co-crystal) of C16H34 shows it to have two-gauche bends.[cite]10.1002/chem.200801428[/cite]. Surprisingly, the longest linear alkane I was able to find a crystal structure for, C28H58 appears to be fully extended[cite]10.1107/S0108768191011059[/cite],[cite]10.1107/S0567740876005025[/cite] (an early report of a low quality structure for C36H74[cite]10.1107/S0365110X5600111X[/cite] also appears to show it as linear).‡ Here I explore how standard DFT theories cope with these structures.