Mention carbon dioxide (CO2) to most chemists and its properties as a metal ligand are not the first aspect that springs to mind. Here thought I might take a look at how it might act as such.
There are up to five binding modes with one metal that one might envisage:
- Bonded interaction with the metal via just one oxygen atom,
- Bonded interaction via just the central carbon atom,
- Bonded interaction via the π-face of one C=O double bond,
- A weaker non-bonded interaction via carbon, or
- via oxygen.
Search queries of the Cambridge structure database (CSD) for these five modes are illustrated below (dataDOI: 10.14469/hpc/2524), with the constraints being applied to how many bonds (of unspecified type) each atom carries, along with no disorder and no errors. Thus query 1 is constrained by 1-coordination on one oxygen, and two on the carbon and other oxygen.
- This query yields four hits: 10.5517/ccvcdq9, 10.5517/cc12nq6n, 10.5517/cc12nq5m, 10.5517/cc12nq4l. The angle subtended at the central carbon of the CO2 ranges from 172-176°, a very modest bending of the linear CO2. There are no examples where the metal is bonded to both oxygens.
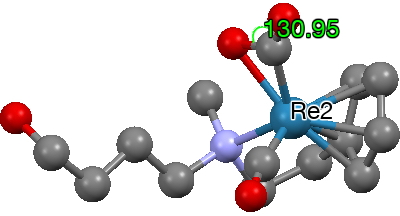
- The next category involves the metal binding just to the central carbon. Two examples are known, differentiated from O-coordination by a more acute angle at the central carbon of 121-132°.
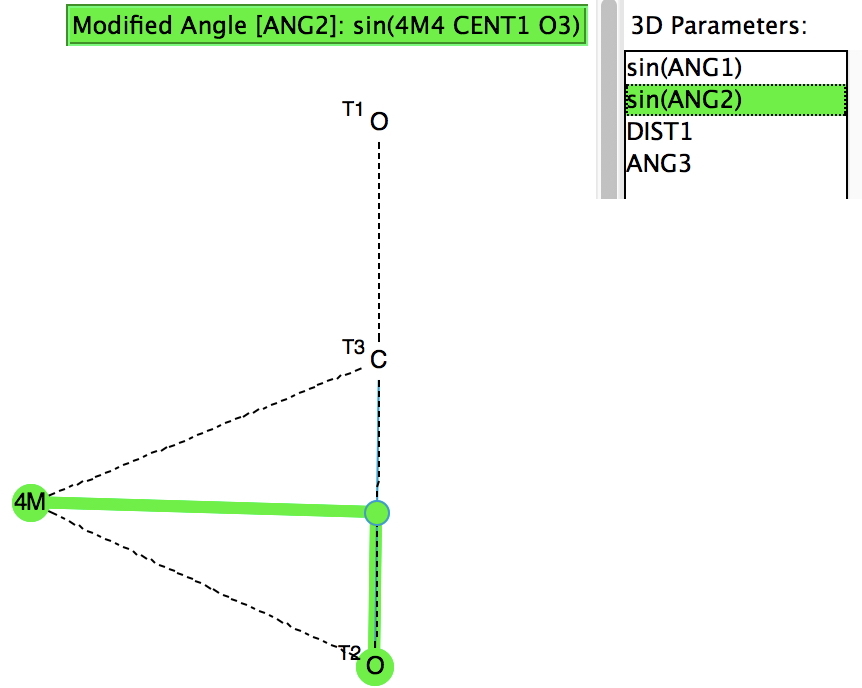
- The π-coordinated type requires a slightly more complex search query, shown below. The π-complex is defined as adding one coordination to each of one oxygen and the carbon.
This reveals 16 examples:
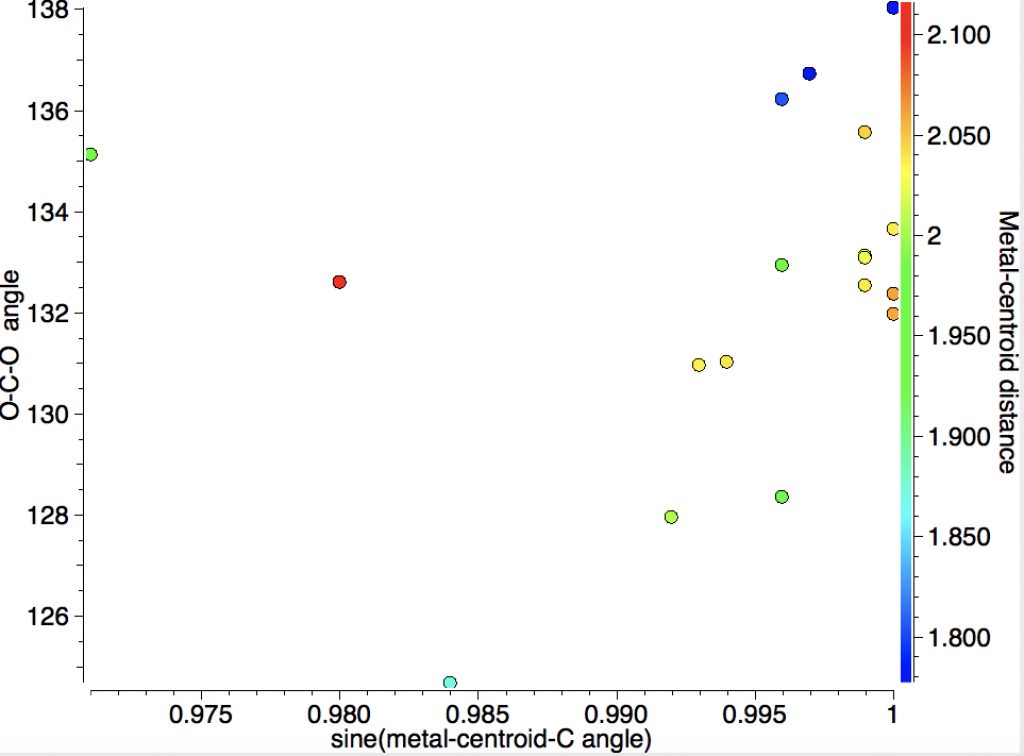
The sine of the angle subtended at the centroid of one C-O bond shows that for most of the examples, the metal is close to perpendicular to this bond. The angle subtended at the central carbon ranges from 128-138, rather larger than the examples where the metal is bound just to the carbon. I have picked these two for illustration. The first (dataDOI: 10.5517/cc86r17) contains both CO2 and CO coordinated to the metal.
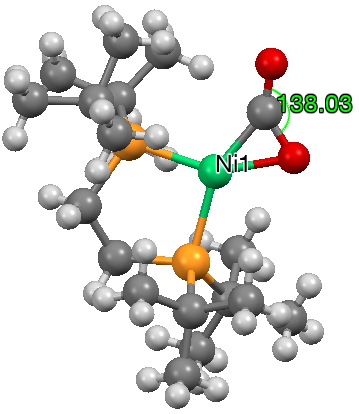
 This one (dataDOI: 10.1021/ic101652e) contains a short metal-centroid distance of 1.78Å (as also does 10.5517/ccz34kr).
This one (dataDOI: 10.1021/ic101652e) contains a short metal-centroid distance of 1.78Å (as also does 10.5517/ccz34kr).
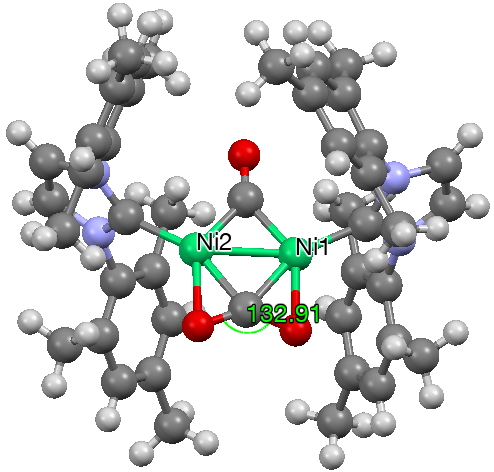
There are two examples where BOTH π-CO bonds are coordinated to a metal; 10.5517/ccqlv7c and 10.5517/ccqlv8d (Ni-centroid distance 1.9Å) but these are intriguing because the two π-complexes are co-planar and not orthogonal.
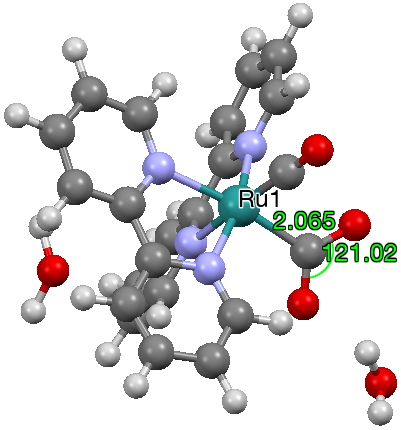
- The final two cases are defined in the CSD database by having not so much bonds between metal and either C or O, as close intermolecular contacts typical of e.g. hydrogen bonds. This one (dataDOI: 10.5517/cc12nq9r) is to Fe, with a metal-C distance of 2.87Å which is significantly shorter than the anticipated sum of the van der Waals radii of the two atoms.
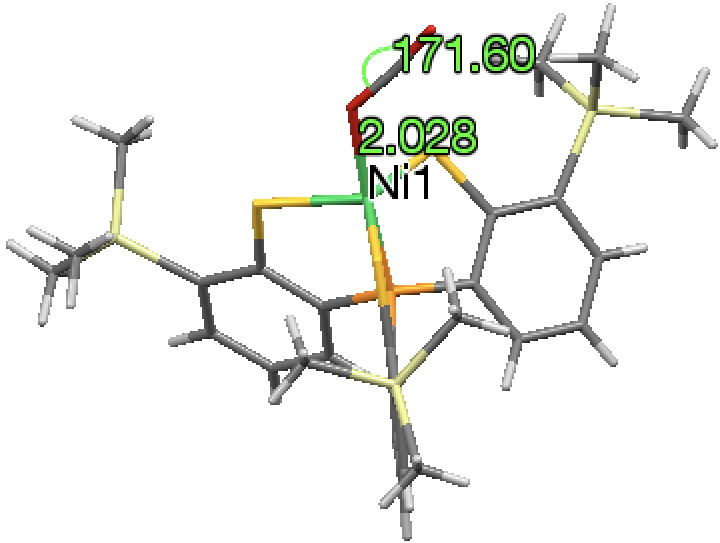
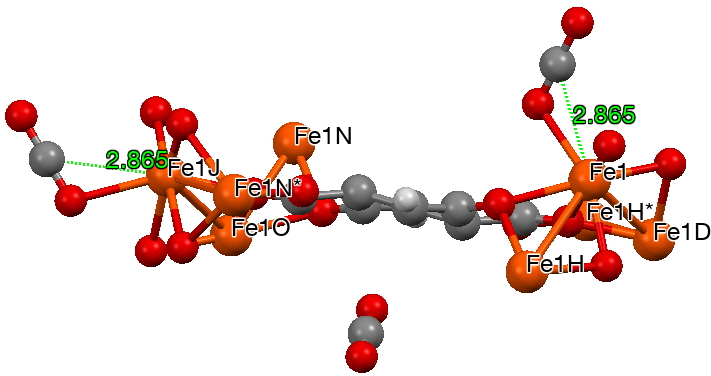 The next (dataDOI: 10.5517/cc12npn2) has a close approach of Co to O of 2.23Å. The angles subtended at the carbon range from 174-180°. There are no convincing examples of close non-bonded approaches of the metal to both oxygen atoms simultaneously.
The next (dataDOI: 10.5517/cc12npn2) has a close approach of Co to O of 2.23Å. The angles subtended at the carbon range from 174-180°. There are no convincing examples of close non-bonded approaches of the metal to both oxygen atoms simultaneously.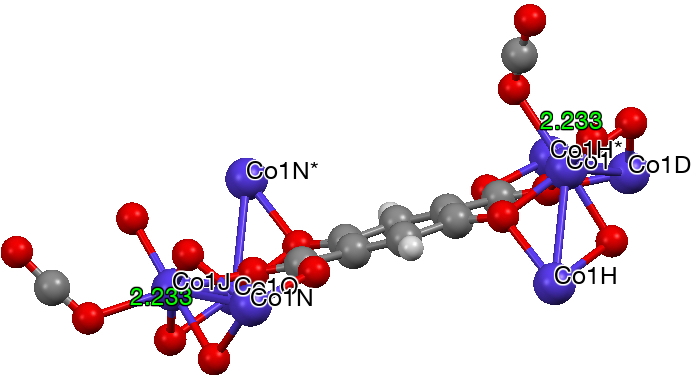
It is striking that the searches (as defined above) reveal relatively few examples. This might simply be a result of how the compounds are indexed in the CSD, reflected in the coordination constraints applied in the searches. Nevertheless, we see three quite different types of ligand-metal coordination in which bonds can be said to form and a more diffuse spectrum of weaker interactions to carbon dioxide. As a metal ligand, it is certainly interesting! Several deserve their wavefunctions looked at and I might report back on this aspect.
Tags: Carbon, Carbon Capture & Storage, carbon dioxide, chemical bonding, Chemistry, Environment, Ligand, ligand-metal coordination, metal, metal ligand, Propellants, Search queries, search query, short metal-centroid distance
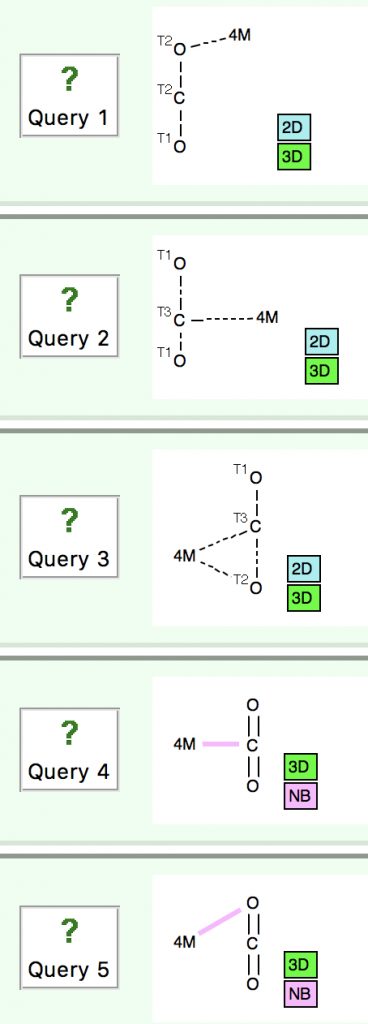
One of my interest points was the structure of the double π-complexed Ni species reported at DOI: 10.1021/ja075630g, noting that the two π-complexes shared the same plane, rather than being orthogonal to each other.
I thought I might check this suggestion out by using allene (H2C=C=CH2) rather than O=C=O. The two double bonds in allene are of course orthogonal to each other and double π-complexes should reflect this. The search query defines centroids for both CC bonds and then charts the torsion angle about the centroid-centroid axis.

You can see from the results that 156 examples of such double π-coordination are known and that the majority have a torsion about the centroid axis of either ~60 or ~90°. Of more interest is that a small number cluster at ~10 (e.g. DOI: 10.1021/om00056a051) and ~180°. The Ni-CO2 complex noted above belongs to the former class, and so is seen as relatively unusual!
More analysis to follow.
Here is an ELF (electron localisation function) analysis:
This shows that the Ni metal atoms do not coordinate in π-complex fashion to each of the two C=O bonds, but instead the interaction is a two-electron-three-centre interaction between the two Ni atoms and the carbon of the CO2, a quite different beast.