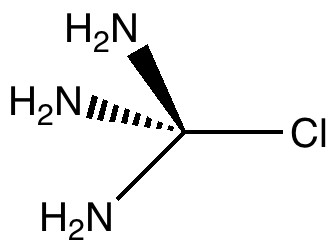
Car transmissions come in two types, ones with fixed ratio gears, and ones which are continuously variable. When it comes to chemical bonds, we tend to think of them as being very much of the first type. Bonds come in fixed ratios; single, aromatic, double, triple, etc. OK, they do vary, but the variations are assumed as small perturbations on the basic form. Take for example the molecule shown below. The bonds as shown are all clearly single (the wedge and hashed bond are merely stereochemical notations). No-one would really think of drawing this molecule in any other way, and this idea of the transferability of bonds between molecules (all double bonds react in specific ways which are different from single bonds, and they also have characteristic spectroscopic properties, etc) is what allows molecules to be classified.
With this molecule however, there really is an elephant in the room; the three electron lone pairs associated with each nitrogen atom (not shown above, but most chemists are trained to recognize their implicit presence). Where are they? Well, each lone pair will tend to orient itself such that it is aligned with an adjacent σ-bond. It has two such bonds to choose from, an adjacent C-N bond or a C-Cl bond. One might now envisage the following permuations; all three N lone pairs gang-up on the C-Cl bond, or perhaps only two do, or only one, or none. What happens in each of these scenarios? The table below shows these permutations calculated using B3LYP/6-31G(d).
| app lone pairs to C-Cl |
Relative free energy, kcal/mol |
C-Cl bond length, Å |
ν C-Cl, cm-1 |
|---|---|---|---|
| 3 | 0.0 | 2.542 | 158 |
| 2 | 4.2 | 2.099 | 221 |
| 1 | 7.3 | 1.937 | 352 |
| 0 | 14.4 | 1.869 | 441 |
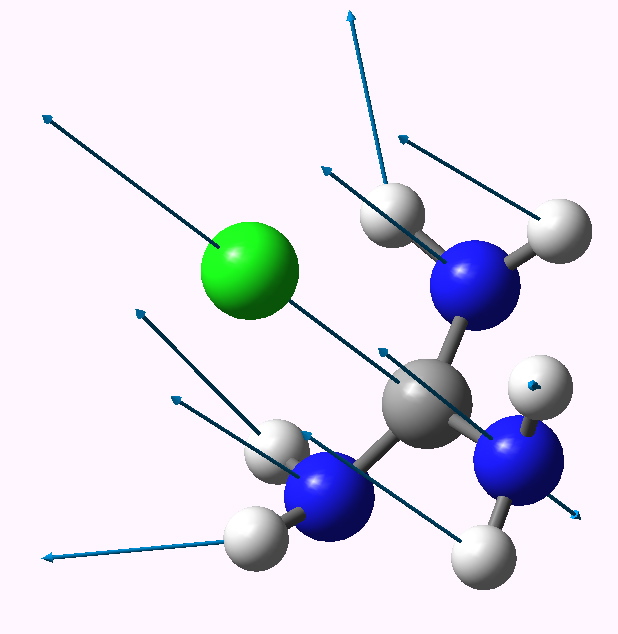 3 app lone pairs. Click for animation |
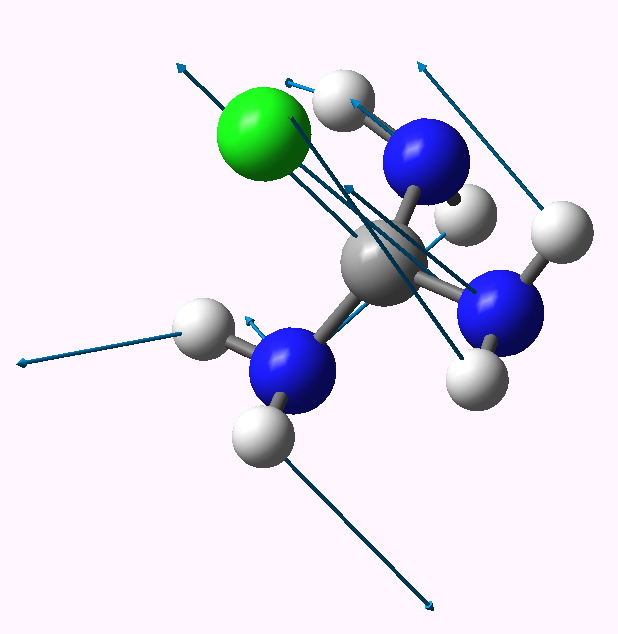 2 app lone pairs. Click for animation |
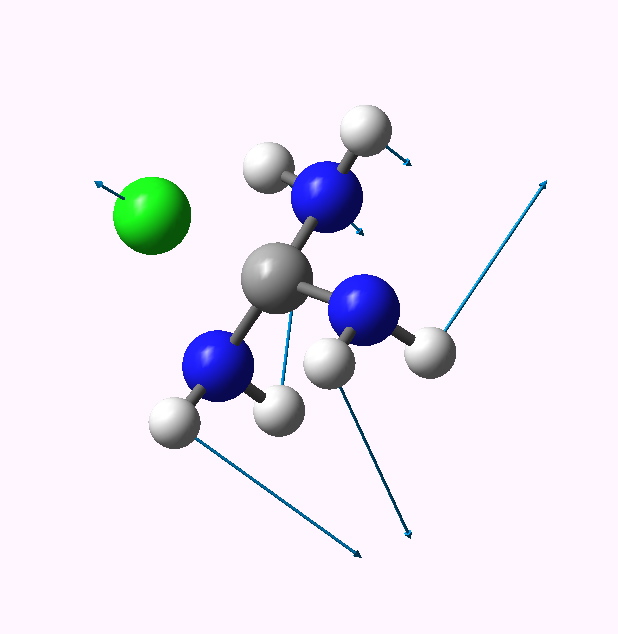 1 app lone pairs. Click for animation |
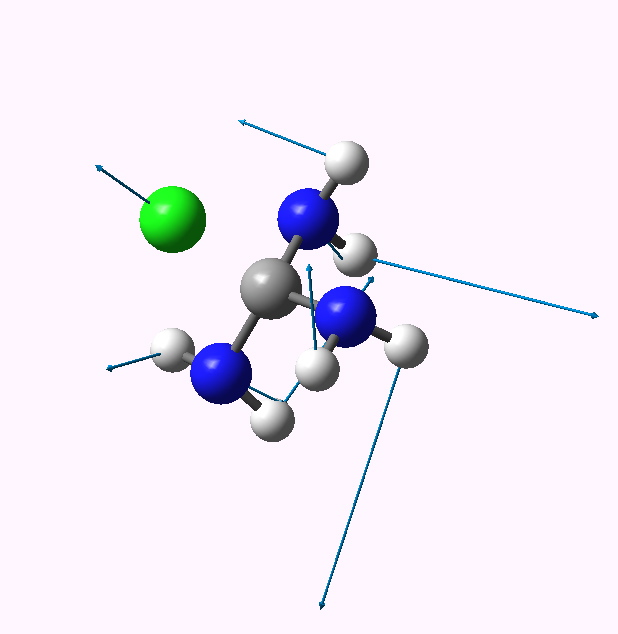 0 app lone pairs. Click for animation |
The C-Cl bond length changes from a normal single bond length (1.87Å) when none of the nitrogen lone pairs are antiperiplanar to the C-Cl bond, to a very abnormal 2.54Å when all three are, and the C-Cl stretching mode decreases in wavenumber from 441 to 158 cm-1. There is lots of other fun to be had inspecting the geometries and vibrations, but I will leave that for you to explore rather than discuss it here. Click on the thumbnails above to start.
This effect does have a name, sugar chemists call it the anomeric effect. But this one is supercharged! It would be quite reasonable to say that at some stage, the C-Cl single bond turns from being covalent to being ionic (and indeed, repeating the calculation using an applied solvent field certainly accelerates this process). Whilst this might be a contrived example and hence an extreme example, it does serve to remind us that on occasion, molecules may come with continuously variable transmissions rather than with fixed ratio gears!
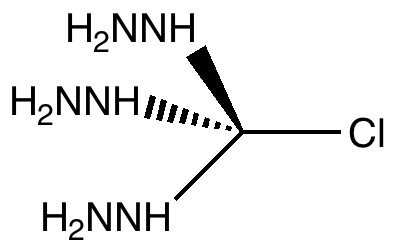
And a postscript. I mentioned the nitrogen lone pairs ganging up on the C-Cl bond. How might one go even one step further? A standard trick to enhance the donating power of a nitrogen lone pair is to replace the NH2 group with a hydrazine group, H2N-NH. The lone pair derived from the second nitrogen buttresses the first. This too has a name, it is called the α-effect.
For this example (see digital repository), the C-Cl bond length lengthens even further to 2.90Å, which interestingly, is the same value as for the SN1 transition state!
Tags: animation, chemical bonds, free energy, H 2 N-NH, hydrazine, Interesting chemistry
A very nice case-study, Henry. You’re right – the discreteness of bond types is yet another thing students have to un-learn as they progress through university.
[…] Henry Rzepa Chemistry with a twist « Tunable bonds […]
[…] bonds looked at in a different way The title of this post merges those of the two previous ones. The tunable C-Cl bond brought about in the molecule tris(amino)chloromethane by anomeric […]