The Willgerodt reaction[1], discovered in 1887 and shown below, represents a transformation with a once famously obscure mechanism. A major step in the elucidation of that mechanism came[2] using the then new technique of 14C radio-labelling, shortly after the atom bomb projects during WWII made 14CO2 readily available to researchers. Here I am going to start the process of applying the far more recent technique of quantitative quantum mechanical modelling to see if some of the proposed mechanisms stand up to its scrutiny.
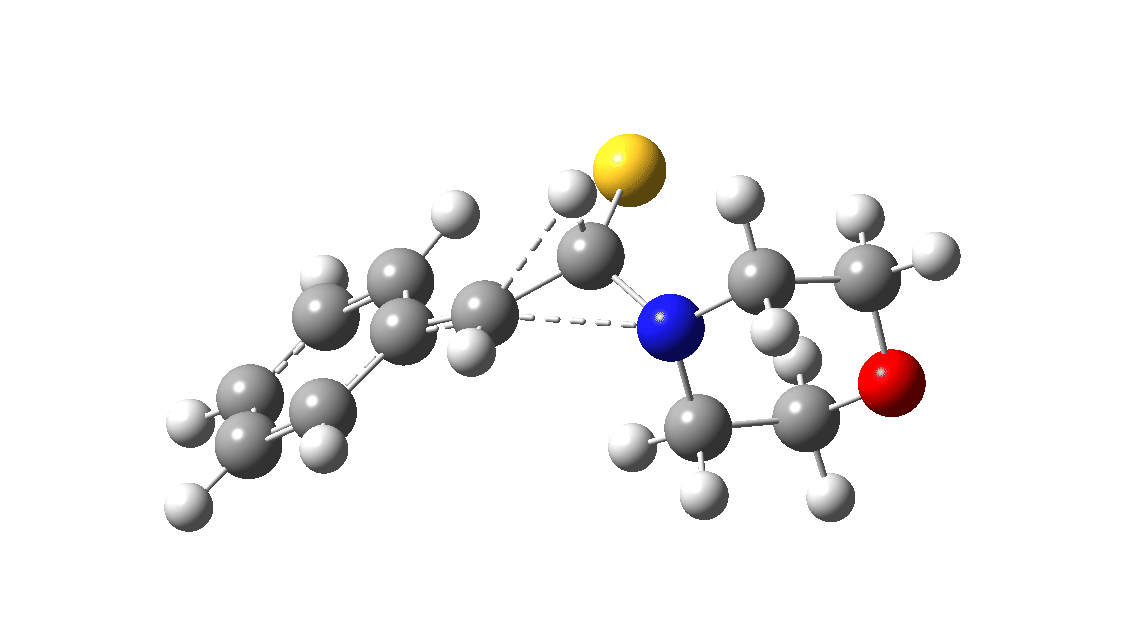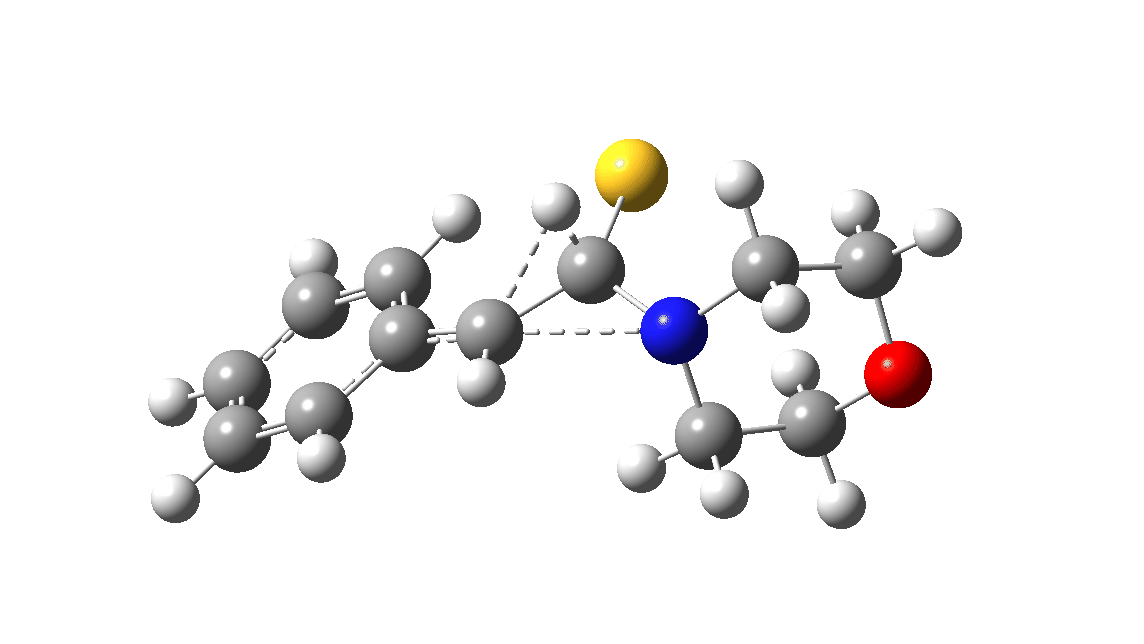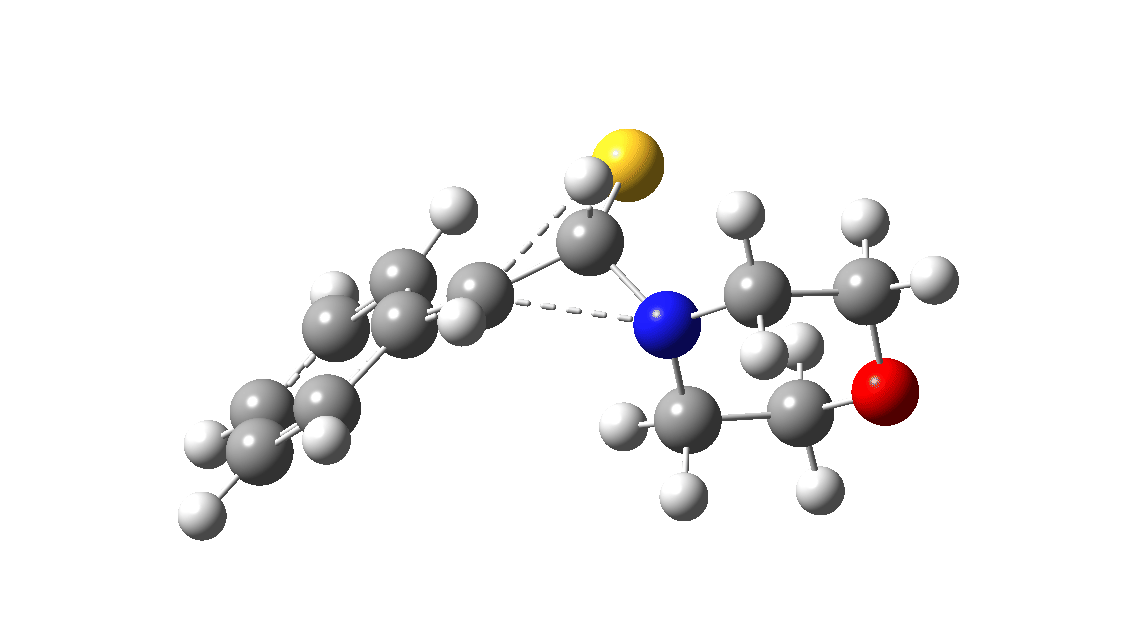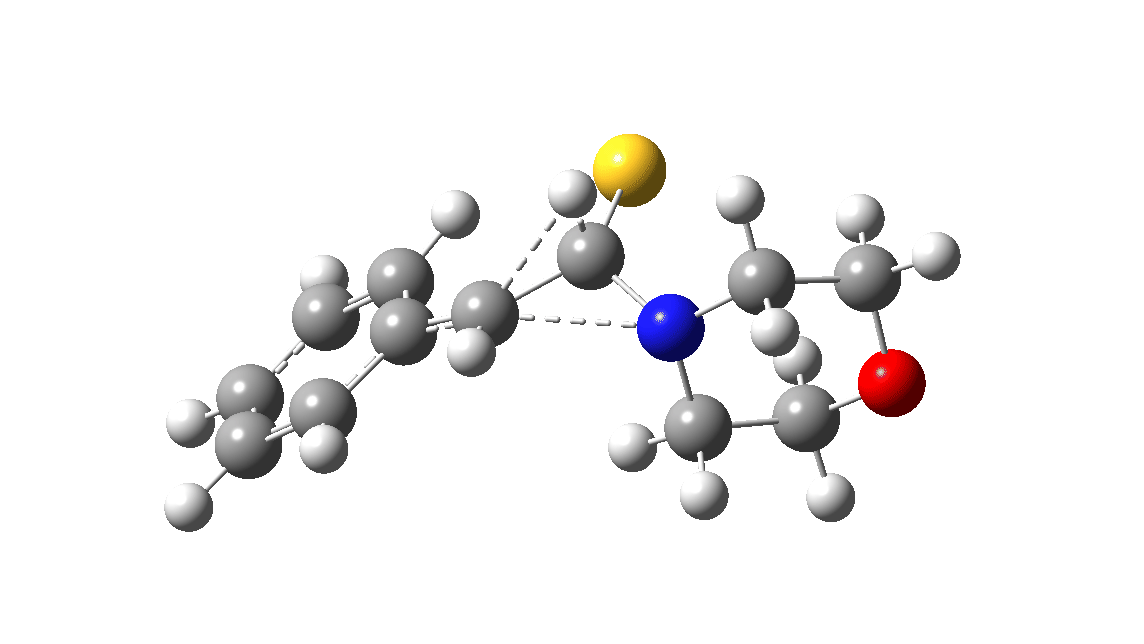
In the classic experiment, it was shown that if acetophenone labelled on the carbonyl group with 14C was subjected to Willgerodt conditions, almost 100% of the label ended up in the benzylic carbon(red dot).[2] Why was this considered remarkable? Because it was effectively the oxygen of the carbonyl (via the proxy of the N in the intermediate enamine) that appeared to be migrating along the C2 carbon chain, rather than the phenyl group which is a known very effective migrator! The rather harsh conditions of the Willgerodt reaction were replaced in 1923 by the somewhat milder Kindler modification,[3] using morpholine + S8 as the catalyst instead of ammonia + S8. The mechanism below is adapted from a typical source of named organic reactions; the Wikipedia page is a rather more elaborate version of this. In essence these mechanisms suggest that the aziridine species labelled Int2 below is an undetected intermediate accounting for the radio-labelling experiment.
The computational reality check can be undertaken by calculating the relative free energies of the species labelled above, setting that of the reactant to ΔΔG = 0.0. The model used is B3LYP+GD3+BJ/Def2-TZVPP/SCRF=water (FAIR data DOI: 10.14469/hpc/7294). For the reaction to be reasonably fast at 403K, the highest species on this pathway should be no higher than ~30 kcal/mol above the reactant. The calculations reveal that TS3 is around 42.6 kcal/mol above the reactant, with a very flat potential energy surface in the region of the transition state, in which C-N cleavage preceeds 1,2-hydrogen migration.
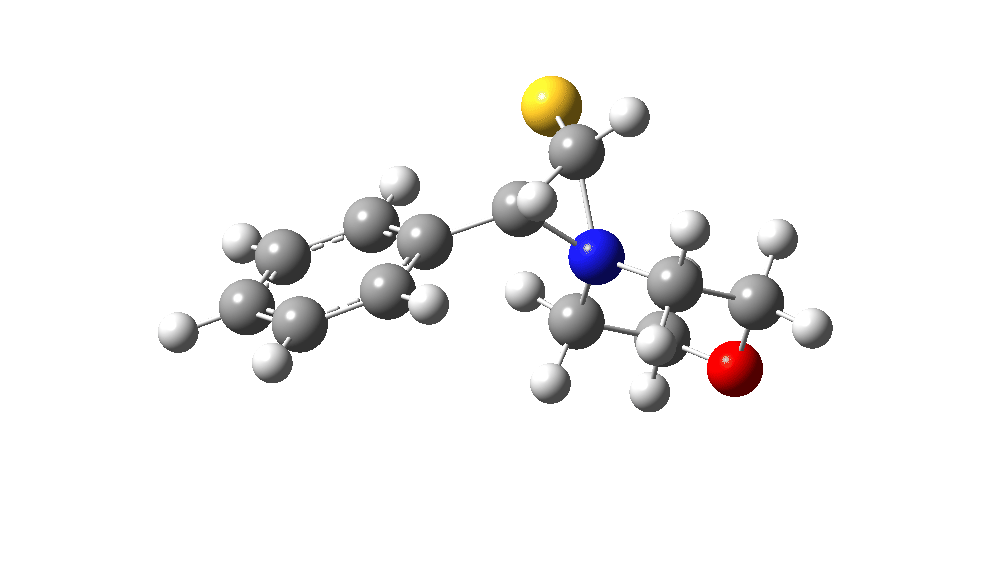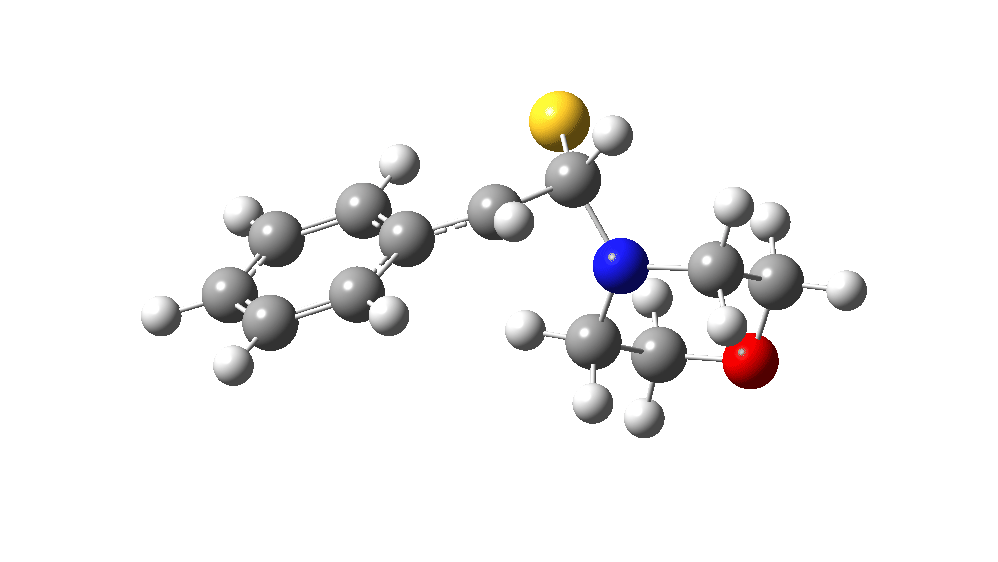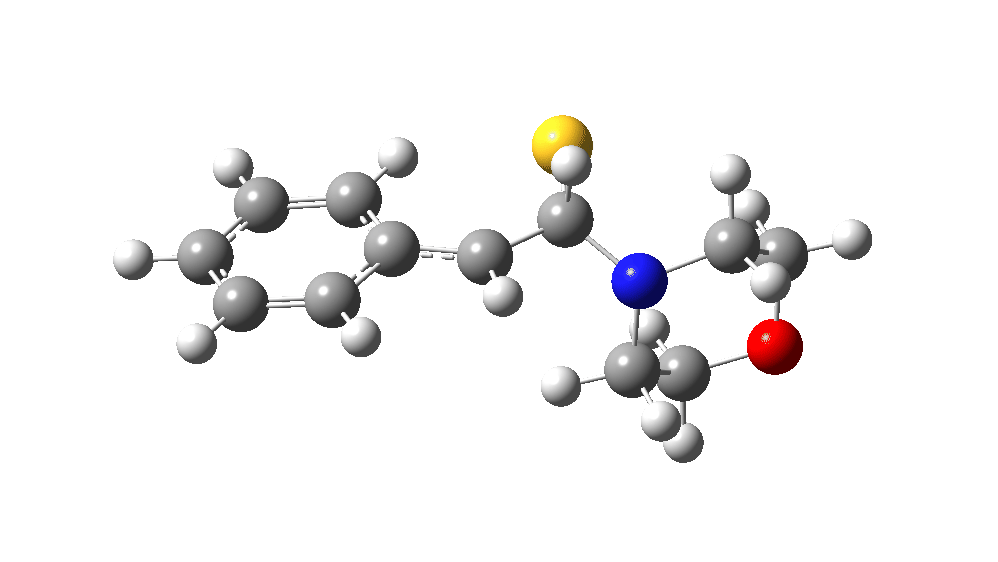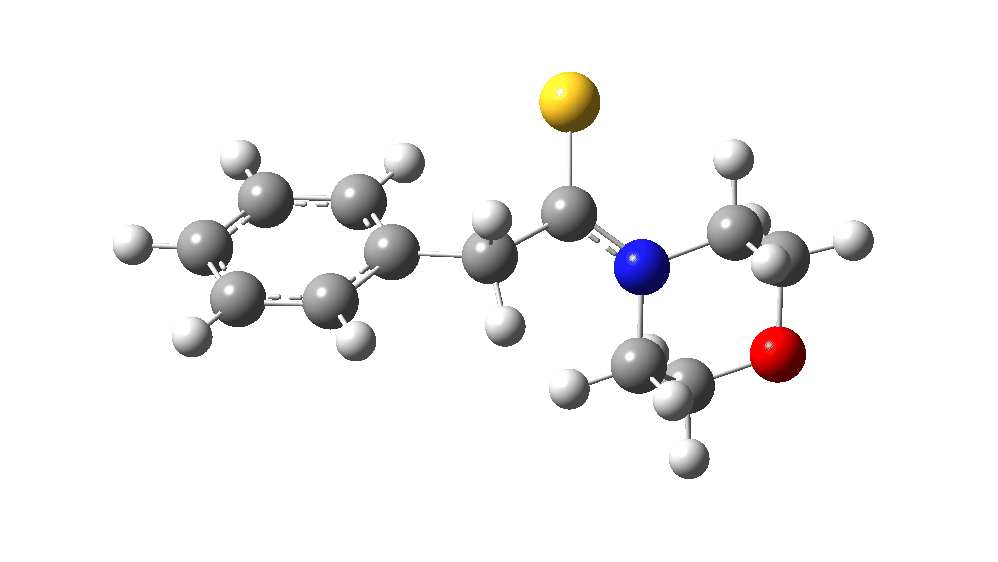
The value of this barrier height suggests an alarm bell ringing. Protonating the species (via tosic acid) does not help. So we conclude that the mechanism needs “optimising” to try to find a lower energy pathway to product.
| Species | ΔΔG |
|---|---|
| Reactant | 0.0 |
| TS1 | 27.9 (44.4)a |
| Int1 | 5.8 (13.0)a |
| TS2 | 16.9 (22.4)a |
| Int2 | 14.7 |
| TS3 | 42.6 |
| Product | -21.2 |
| Int3 | -0.4 |
| aProtonated on S | |
A hint of how this might be done comes from the energy of the species labelled Int3, which is a thiirane rather than an aziridine intermediate. Such thiiranes will be explored in part two of this theme. It may also be that explicit base catalysis of TS3 via proton abstraction may be more facile than a direct [1,2] hydrogen shift. Much like organic syntheses, where reaction yields have to be optimised by often long and arduous explorations, so too on occasion do reaction mechanisms!
References
- C. Willgerodt, "Ueber die Einwirkung von gelbem Schwefelammonium auf Ketone und Chinone", Berichte der deutschen chemischen Gesellschaft, vol. 20, pp. 2467-2470, 1887. https://doi.org/10.1002/cber.18870200278
- W.G. Dauben, J.C. Reid, P.E. Yankwich, and M. Calvin, "The Mechanism of the Willgerodt Reaction<sup>1</sup>", Journal of the American Chemical Society, vol. 72, pp. 121-124, 1950. https://doi.org/10.1021/ja01157a034
- K. Kindler, "Studien über den Mechanismus chemischer Reaktionen. Erste Abhandlung. Reduktion von Amiden und Oxydation von Aminen", Justus Liebigs Annalen der Chemie, vol. 431, pp. 187-230, 1923. https://doi.org/10.1002/jlac.19234310111
Interesting work. How can you get the IRC to work of the extremely flat area? My calculations always stop after 2~3 points in such scenario during IRC calculations. It would be very nice of you if you could just respond. Thanks in advance.
Wendy,
The easiest way of finding out the answer to your question is to go visit the FAIR data I have made available in the post (https://doi.org/10.14469/hpc/7294) and take a look at some of the input files. These will show the keywords used to produce a full IRC.
Cheers
Henry
Henry,
I found it. Thanks a lot.
Best regards,
Wendy
Hi Henry,
I came across you blog many years ago and enjoy reading your studies. I’m working on a named reaction website, and the mechanism of the Willgerodt-Kindler reaction always bothered me. Carmack’s thiirene intermediate seemed too energetic and your calculations support that. I came up with another one that involves thiirane and aziridine intermediates and which can explain the migration of the C=N bond across the backbone. I can send you a pdf of the scheme. I’m very curious what you think of it.
Best,
Mike
Thanks Mike. Have you checked DOI: https://doi.org/10.59350/g9kgn-z4915 (completing the box set) as well as https://doi.org/10.59350/r703q-tz017 https://doi.org/10.59350/exggd-ztz94 and the one you comment on, https://doi.org/10.59350/nh8q6-7k182
I am sure other pathways are feasible and I encourage you to explore them. If you want to share your model here, do send the PDF and I will add it to your comment.
Thank you for the reply! I missed the complete box set. The one I have is conceptually similar as it invokes both the thiirane and the aziridine intermediates to explain the “iminium walk”. I use the simple thiol rather than S-Mor to avoid the positively charged =S(+)Mor intermediate. I like your termination sequence, and prefer it from what I have now. You can check my mech scheme at: https://say-my-name.net/advanced?letter=W