In the earlier post on the topic of anomeric effects, I identified a number of outliers associated with large differences in the lengths of two carbon-oxygen bonds sharing a common carbon atom.
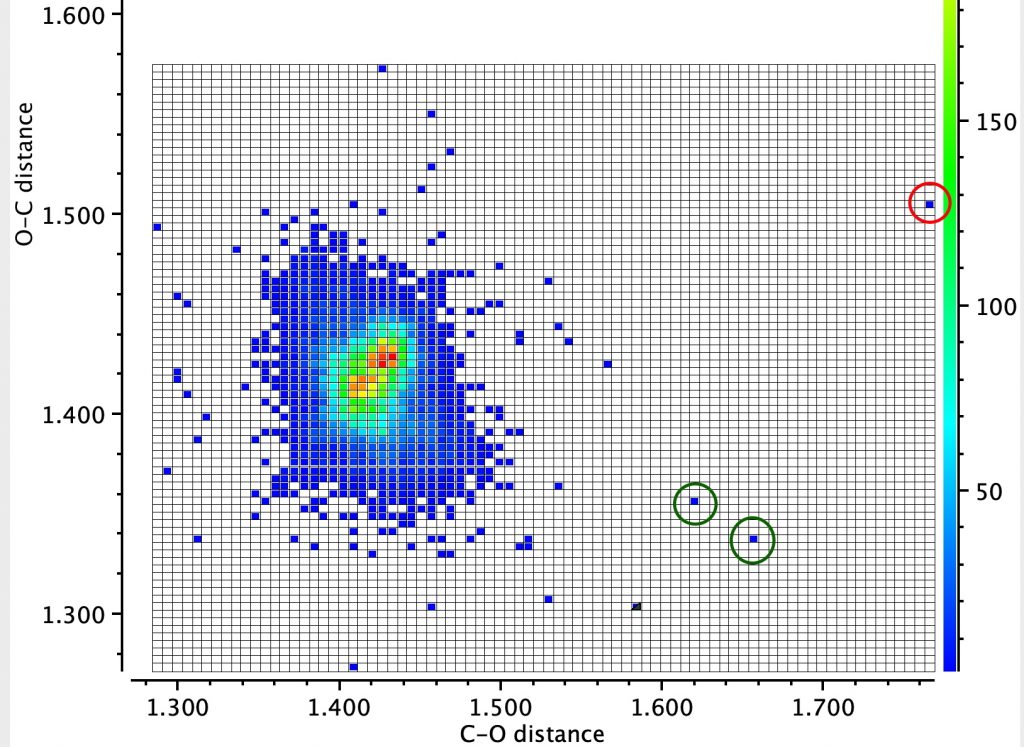
Here is another of these outliers (MUZZIS[1]) which shows equally unusual properties. This is an oxyanion (counterion is trimethylbenzylammonium) which is part of a very strong O-H-O hydrogen bond in which the O…O distance is 2.44Å and the O-H distances are each indicated as 1.23Å, suggesting the hydrogen is symmetrically disposed about the two oxygens. The anomerically lengthened C-O bonds (shown in red below) are 1.513/1.516Å, which is indeed long for a C-O bond.
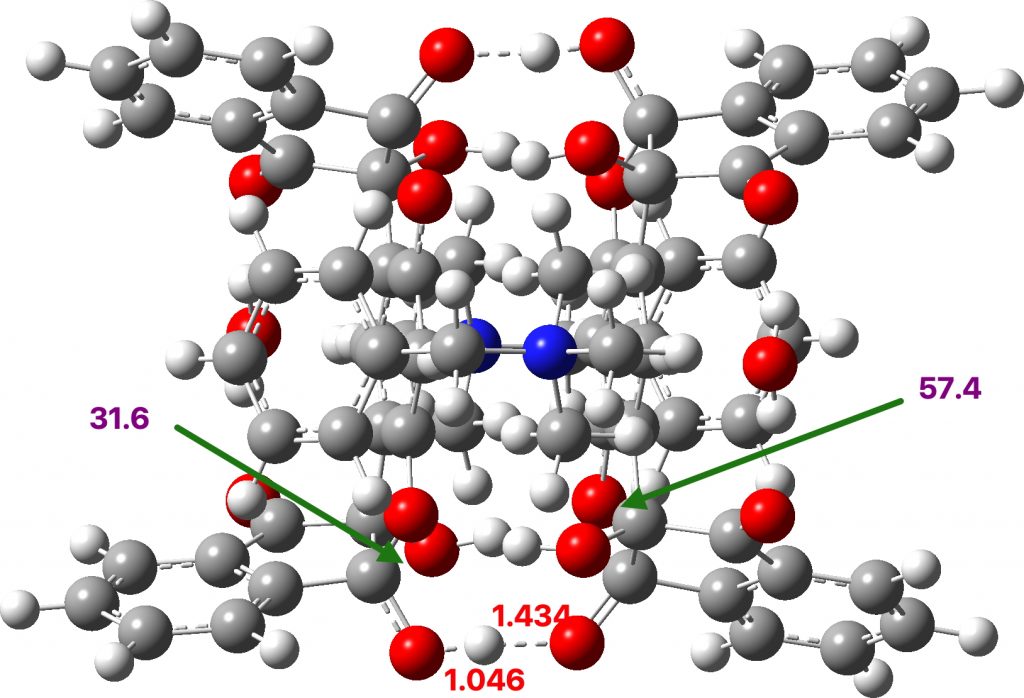
A ωB97XD/Def2-SVPP/SCRF=chloroform calculation reveals a less symmetrical hydrogen bonding system, with calculated O-H distances of 1.046/1.434 (mean 1.24Å) and an O-O distance of 2.48Å. The anomerically lengthened C-O distances are 1.479/1.582Å. There are several reasons for these differences:
- The temperature at which the X-ray data were recorded was 173K and it remains possible that the data represent an averaged position for the atoms at this temperature rather than a truly symmetrical hydrogen bond.
- The basis set/DFT method for the calculation may itself favour unsymmetrical hydrogen bonds.
The two NBO energy perturbation terms for one lone pair on the oxygens interacting with the empty C-O σ* orbital are 31.6 and 57.5 kcal/mol. If the hydrogen bond is in reality entirely symmetric, then the NBO term would be expected to be approximately the average of these two values.
Click image to obtain 3D model
The earlier record anomeric holder achieved this by virtue of charge relocation involving an oxenium cation rather than the normal effect of charge separation. Here we have a similar effect, this time involving oxy-anionic charge relocation. Both are therefore special cases.
References
- R. Bengiat, M. Gil, A. Klein, B. Bogoslavsky, S. Cohen, and J. Almog, "Bis(benzyltrimethylammonium) bis[(4<i>SR</i>,12<i>SR</i>,18<i>RS</i>,26<i>RS</i>)-4,18,26-trihydroxy-12-oxido-13,17-dioxaheptacyclo[14.10.0.0<sup>3,14</sup>.0<sup>4,12</sup>.0<sup>6,11</sup>.0<sup>18,26</sup>.0<sup>19,24</sup>]hexacosa-1,3(14),6,8,10,15,19,21,23-nonaene-5,25-dione] sesquihydrate: dimeric structure formation<i>via</i>[O—H—O]<sup>−</sup><i>negative charge-assisted hydrogen bonds (–CAHB)</i>with benzyltrimethylammonium counter-ions", Acta Crystallographica Section E Crystallographic Communications, vol. 72, pp. 399-402, 2016. https://doi.org/10.1107/s2056989016002899