Calicheamicin is a natural product with antitumour properties discovered in the 1980s, with the structure shown below. As noted elsewhere, this structure has many weird properties, including amongst other features an unusual “enedidyne” motif and the presence of an iodo group on an aromatic ring. Its isolated 3D structure is quite difficult to get hold of (embedded structures in a DNA fragment are available however); the 3D model associated with the Wikipedia entry is essentially only in 2D. The representation shown below, including the absolute stereochemistry, was obtained from the SciFinder entry.
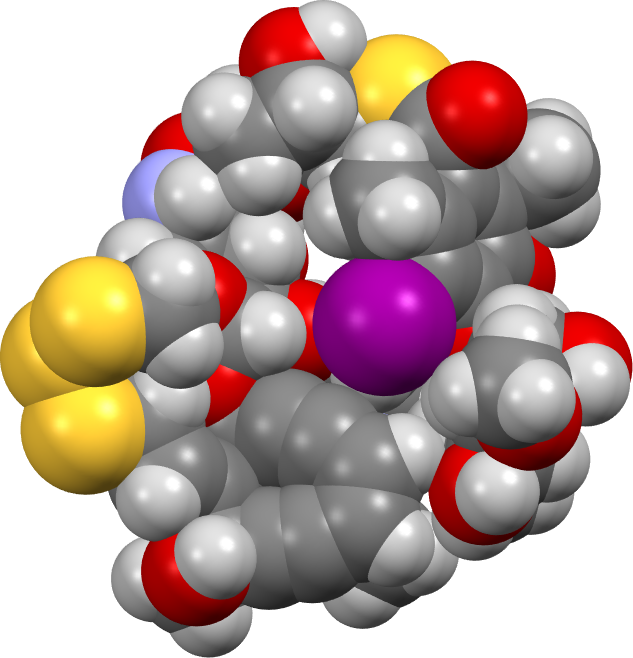
As a prelude to modelling the mechanism of the Bergman cyclisation (for Part 1 in which a simple cycloendiyne is explored, see DOI: 10.59350/jczra-f0r90 [1]) of the enediyne ring on this actual molecule, a 3D model was constructed.‡ One possible such model is shown below, built to maximise wherever possible interactions such as hydrogen bonds and weak dispersion attractions from eg methyl groups. A side benefit of doing this is the natural emergence of a “cavity” in which the very large iodine atoms snuggles, as it happens adjacent to the enediyne component – something you would not naturally infer from the structure representation shown above! A spacefill model of this conformation is shown below (click on the image to get an interactive version), emerging from an ωB97XD/Def2-SVPP energy minimisation (DOI: 10.14469/hpc/14586).[2]
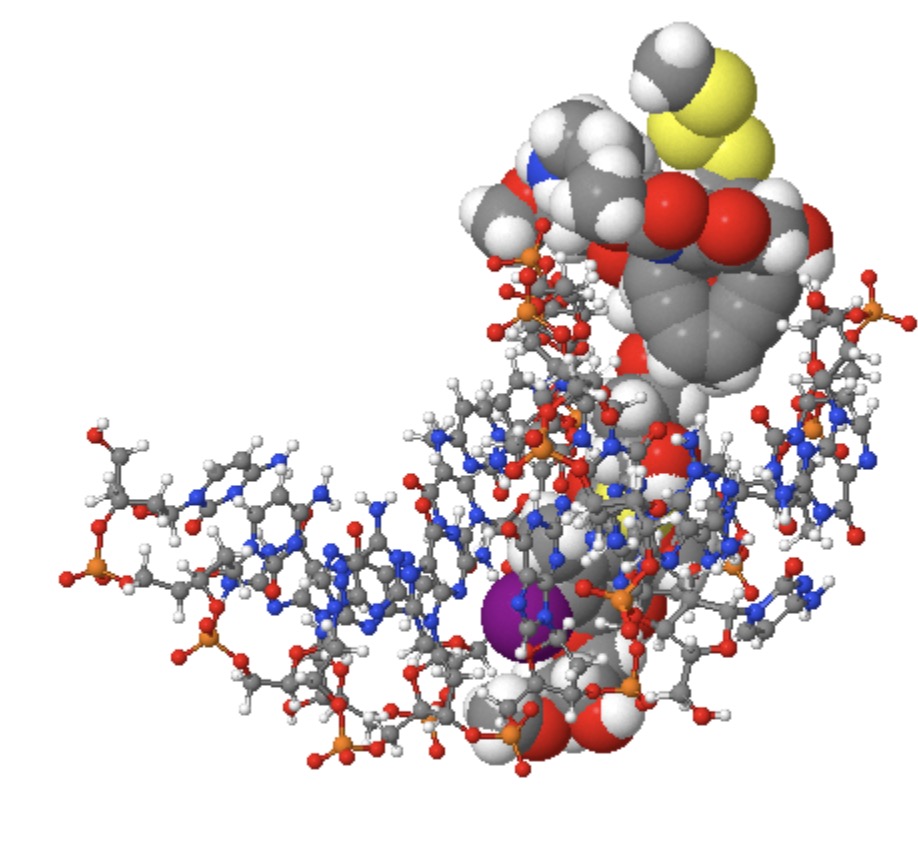
The below shows a crystal structure (2pik) of Calicheamicin embedded into a DNA duplex,† which shows a stretched linear conformation of Calicheamicin rather than the compact form more appropriate for an isolated molecule.
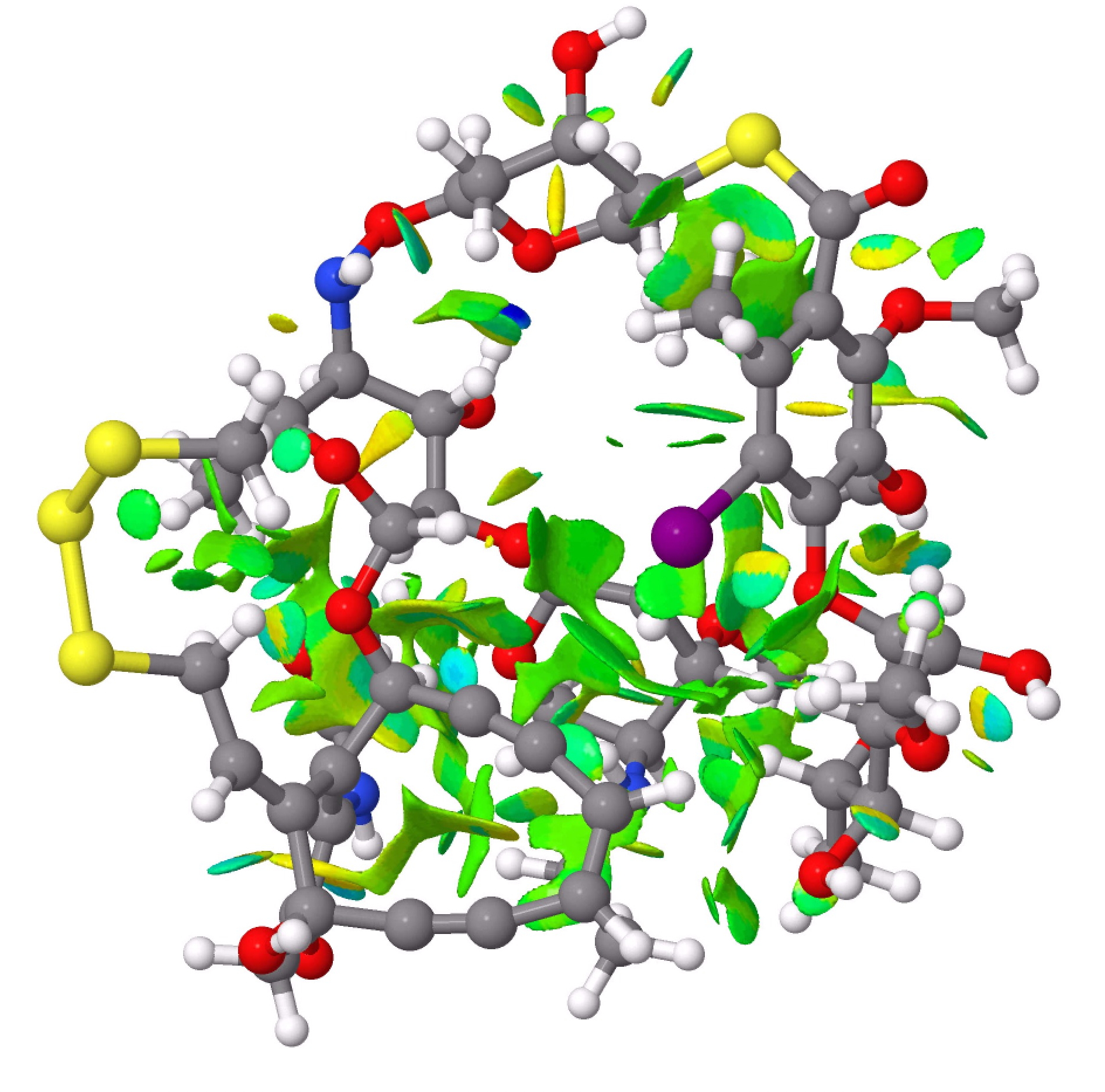
The next step was to use the ωB97XD/Def2-SVPP wavefunction[2] to calculate the full electron density for the molecule, and using this to evaluate the NCI (non-covalent-interaction) isosurfaces. These are shown below, and the eye is immediately drawn to the regions surrounding that iodine atom, which are replete with attractive green surfaces. Blue and cyan coloured surfaces derive from hydrogen bonds formed within the 3D structure (click on the image to get an interactive version, but be patient, it takes a little while to load).
The next stage, using the model to evaluate the energetics of the Masamune-Bergman cyclisation for Calicheamicin itself will be reported in part 3.
‡For those interested, this was constructed in stages. The structure representation had been drawn in Chemdraw, saved as a pseudo 3D molfile and then loaded into Gaussview. There, it was subjected to several cycles of energy minimisation using the MMFF94 molecular mechanics force field. The stereochemistry of all the centres was carefully checked at each stage, if necessary corrected and re-optimised. The next stage was to subject it to a PM7 semiempirical SCF minimisation, a method which includes dispersion attraction terms and which tends to give geometries that are quite close to eg those obtained using dispersion-corrected DFT methods, in this example ωB97XD/Def2-SVPP.
References
- H. Rzepa, "Mechanism of the Masamune-Bergman reaction. Part 1.", 2024. https://doi.org/10.59350/jczra-f0r90
- H. Rzepa, "Calicheamicin, full system, reactant, wB97XD/Def2-svpp G = -5768.785232", 2024. https://doi.org/10.14469/hpc/14586