Each year C&E News publishes a list of candidates for the Molecule of the Year. For 2024 the list is (in order of votes cast for each)
- Mirror-image cyclodextrin [1]
- Molecular shuttle in a box [2]
- Rule-bending strained alkene [3]
- First soluble promethium complex [4]
- Single-electron carbon-carbon bond [5]
- Hot MOF for capturing carbon[6]
I dealt at length with entry 5 (single-electron carbon-carbon bond) last year, my conclusions rather negating the statement made about it being an example of such a bond. Here I take a look at number 3, A solution to the anti-Bredt olefin synthesis problem.[3] Four molecules below (1-4) were identified as examples of anti-Bredt rule compounds from trapping experiments (their properties such as NMR or indeed structures are not reported). Julius Bredt had predicted 100 years ago would be particularly unstable.[7]
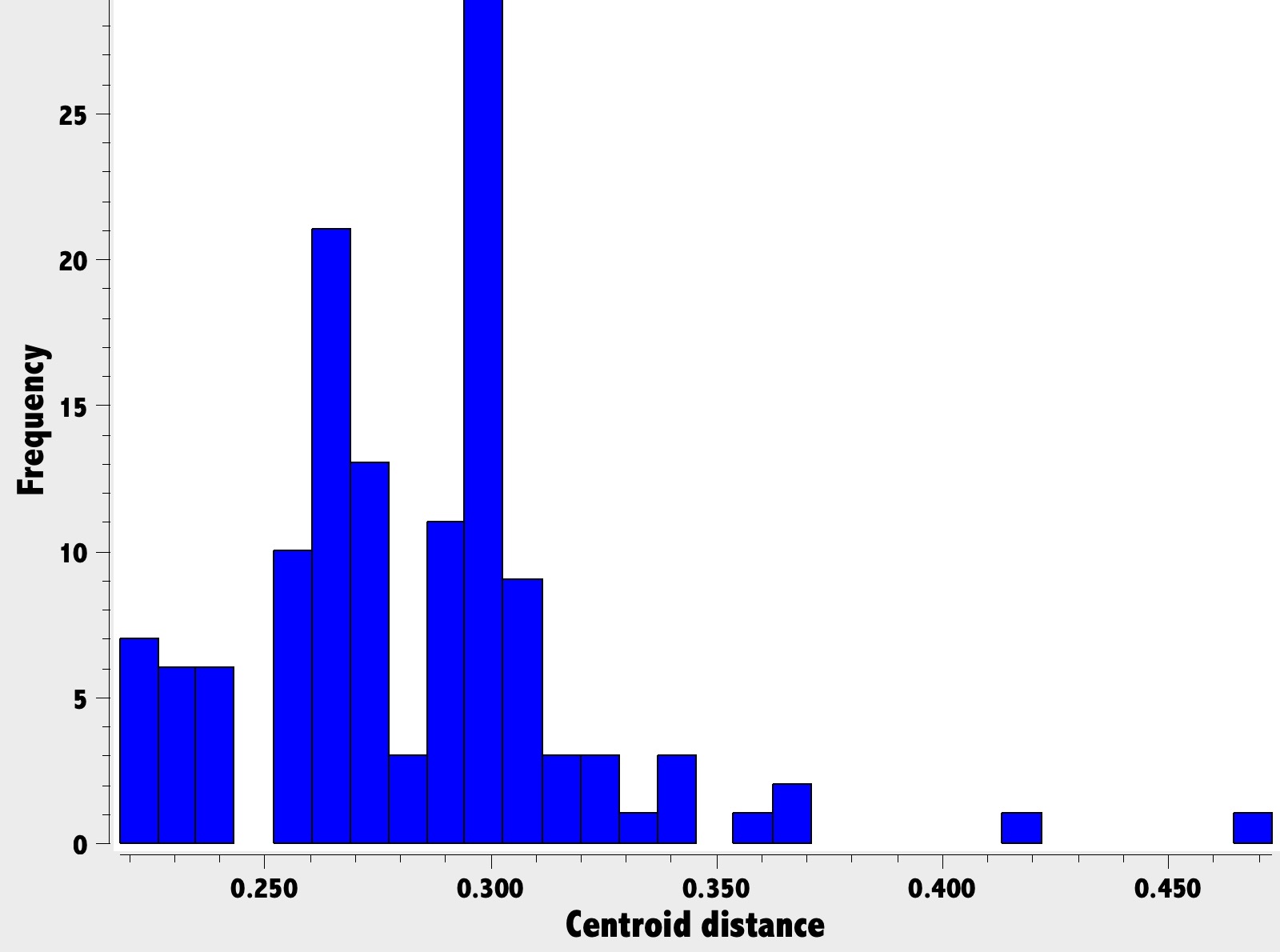
One way of putting these molecules into context is to search for any similarly strained alkenes in the Cambridge crystal database. The search query used defined a centroid of the plane defined by the three carbon atoms attached to the bridgehead carbon atom, and then the distance from that centroid to the carbon atom itself. For entirely planar coordination of that atom, the distance would be ~zero and the deviation from zero is one way of measuring how strained the alkene is.
The results of the search (for which fullerenes are excluded as special cases) is shown below. The upward limits of the centroid distance are between ~0.3 – 0.34Å; the outlier at 0.47Å appears to be an error, since the corresponding C=C distance is 1.565Å.
Screenshot
For comparison, the centroid distance to four-coordinate carbon (a central carbon with four attached carbon ligands) is shown below – the most probable value being ~0.51Å.
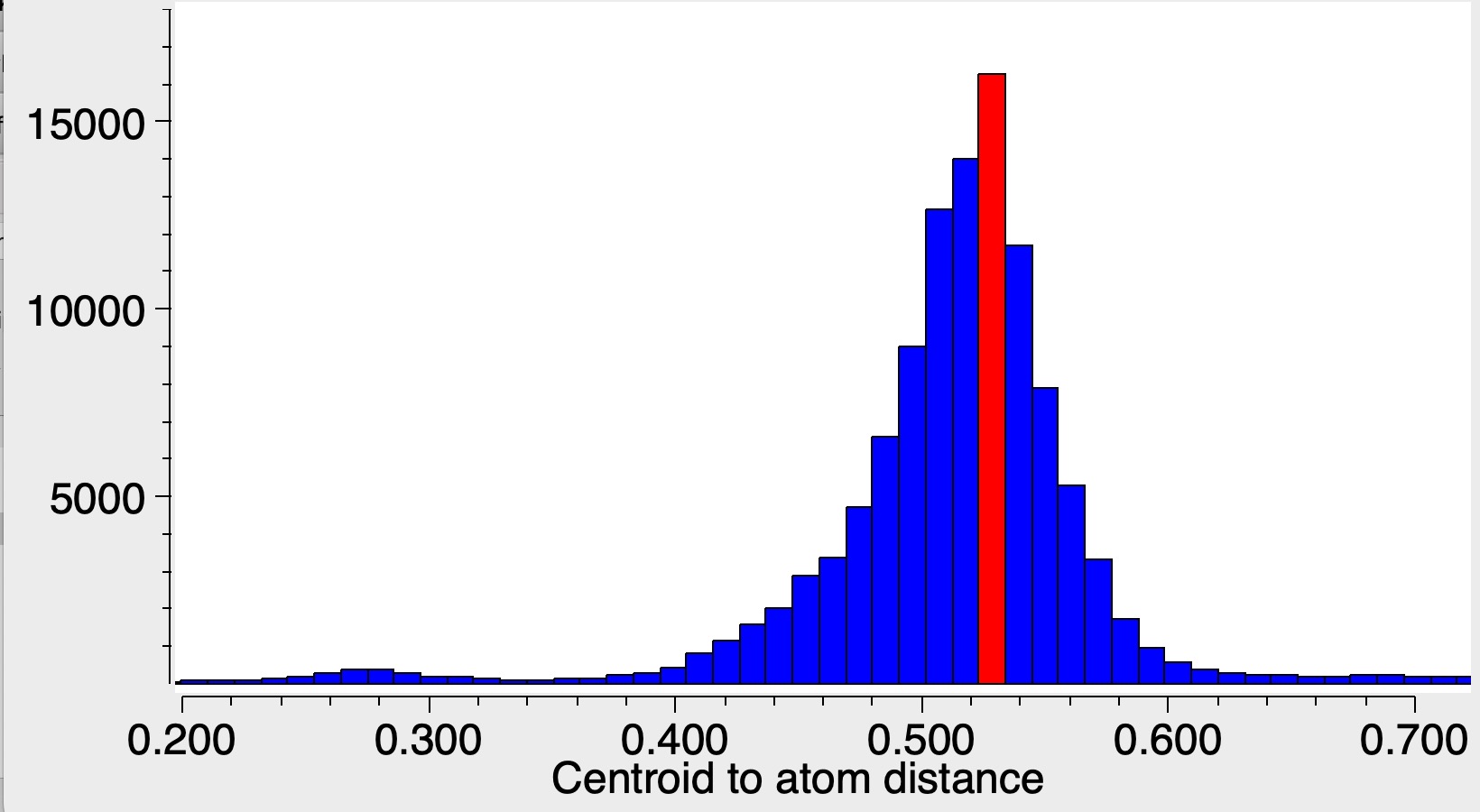
Screenshot
Since compounds 1-4 were not actually isolated, no crystal structures or NMR data are available. ωB97XD/Def2-TZVPP calculations were performed to establish trends in these properties (FAIR Data [8]).
| Molecule | Centroid distance, Å | C=C length | ν cm-1 | δ 1H | δ 13C |
|---|---|---|---|---|---|
| 1 (“ABO 12”) | 0.510 (adduct 62 [3]) | 1.346 | 1611 | 6.76 | 189.8 |
| 2 | 0.505 (adduct 58 [3]) | 1.349 | 1594 | 6.76 | 196.8 |
| 3 | 0.341 (adduct 72 [3]) | 1.341 | 1684 | 5.84 | 170.3 |
| 4 | 0.357 (adduct 68 [3]) | 1.336 | 1694 | 6.26 | 173.3 |
For compounds 1-2, the largest ring of the three associated with the bridgehead carbon is six, whereas for compounds 3-4 it is seven. This is reflected in the values shown in the table above. The centroid distance for the six-ring examples is close to 0.5Å, for which no examples exist in the crystal structure database. The centroid distance for the seven-ring examples is 0.34-0.35Å, for which a number of crystalline examples are evident. It seems likely then that compounds 3-4 stand a better chance of being isolated as such, rather than having their existence inferred from the cycloadducts they form. Perhaps a modification to the experimental procedures might accomplish this? The predicted 1H and 13C spectra are shown in the table to aid identification if this is ever achieved. Also noteworthy are the C=C stretching vibrations, which are lowered significantly for 1-2 compared to 3-4.
Its good to have experimental evidence for compounds that 100 years ago were predicted to be unusually unstable. Perhaps the next step is to isolate them as pure compounds and study their properties.
References
- Y. Wu, S. Aslani, H. Han, C. Tang, G. Wu, X. Li, H. Wu, C.L. Stern, Q. Guo, Y. Qiu, A.X. Chen, Y. Jiao, R. Zhang, A.H.G. David, D.W. Armstrong, and J. Fraser Stoddart, "Mirror-image cyclodextrins", Nature Synthesis, vol. 3, pp. 698-706, 2024. https://doi.org/10.1038/s44160-024-00495-8
- S. Ibáñez, P. Salvà, L.N. Dawe, and E. Peris, "Guest‐Shuttling in a Nanosized Metallobox", Angewandte Chemie International Edition, vol. 63, 2024. https://doi.org/10.1002/anie.202318829
- L. McDermott, Z.G. Walters, S.A. French, A.M. Clark, J. Ding, A.V. Kelleghan, K.N. Houk, and N.K. Garg, "A solution to the anti-Bredt olefin synthesis problem", Science, vol. 386, 2024. https://doi.org/10.1126/science.adq3519
- D.M. Driscoll, F.D. White, S. Pramanik, J.D. Einkauf, B. Ravel, D. Bykov, S. Roy, R.T. Mayes, L.H. Delmau, S.K. Cary, T. Dyke, A. Miller, M. Silveira, S.M. VanCleve, S.M. Davern, S. Jansone-Popova, I. Popovs, and A.S. Ivanov, "Observation of a promethium complex in solution", Nature, vol. 629, pp. 819-823, 2024. https://doi.org/10.1038/s41586-024-07267-6
- T. Shimajiri, S. Kawaguchi, T. Suzuki, and Y. Ishigaki, "Direct evidence for a carbon–carbon one-electron σ-bond", Nature, vol. 634, pp. 347-351, 2024. https://doi.org/10.1038/s41586-024-07965-1
- R.C. Rohde, K.M. Carsch, M.N. Dods, H.Z.H. Jiang, A.R. McIsaac, R.A. Klein, H. Kwon, S.L. Karstens, Y. Wang, A.J. Huang, J.W. Taylor, Y. Yabuuchi, N.V. Tkachenko, K.R. Meihaus, H. Furukawa, D.R. Yahne, K.E. Engler, K.C. Bustillo, A.M. Minor, J.A. Reimer, M. Head-Gordon, C.M. Brown, and J.R. Long, "High-temperature carbon dioxide capture in a porous material with terminal zinc hydride sites", Science, vol. 386, pp. 814-819, 2024. https://doi.org/10.1126/science.adk5697
- J. Bredt, "Über sterische Hinderung in Brückenringen (Bredtsche Regel) und über die <i>meso</i>‐<i>trans</i>‐Stellung in kondensierten Ringsystemen des Hexamethylens", Justus Liebigs Annalen der Chemie, vol. 437, pp. 1-13, 1924. https://doi.org/10.1002/jlac.19244370102
- H. Rzepa, "Molecules of the Year - 2024. A crystal structure perspective on anti-Bredt olefins.", 2025. https://doi.org/10.14469/hpc/14898
Or maybe the next step is to twist the bond so strongly that forms a “Carbon-Carbon Double Bonds with a Covalent σ and an Ionic p-Bond in Anti-Bredt Olefins”? 🙂
I hope you like our upcoming paper that has been under review for a long time:
https://chemrxiv.org/engage/chemrxiv/article-details/673efa9d7be152b1d0145984