This is another in the C&E News list of candidates for the Molecule of the Year, Molecular shuttle in a box [1]
- Mirror-image cyclodextrin [2]
- Molecular shuttle in a box [1]
- Rule-bending strained alkene [3]
- First soluble promethium complex [4]
- Single-electron carbon-carbon bond [5]
- Hot MOF for capturing carbon[6]
The molecule shown below inside the cavity is coronene. A free energy barrier of ~13 kcal/mol was determined using NMR peak coalescence temperatures, and inferred to correspond to the energy required to move the coronene from one end of the cavity to the other. Here I perform a simple reality check on this result using ωB97XD/Def2-SVP DFT calculations.[7],[8],[9]. This functional includes a second generation dispersion correction, which is the primary effect controlling the position of the coronene inside the cavity.
Firstly, the fully optimised geometry of the complex.
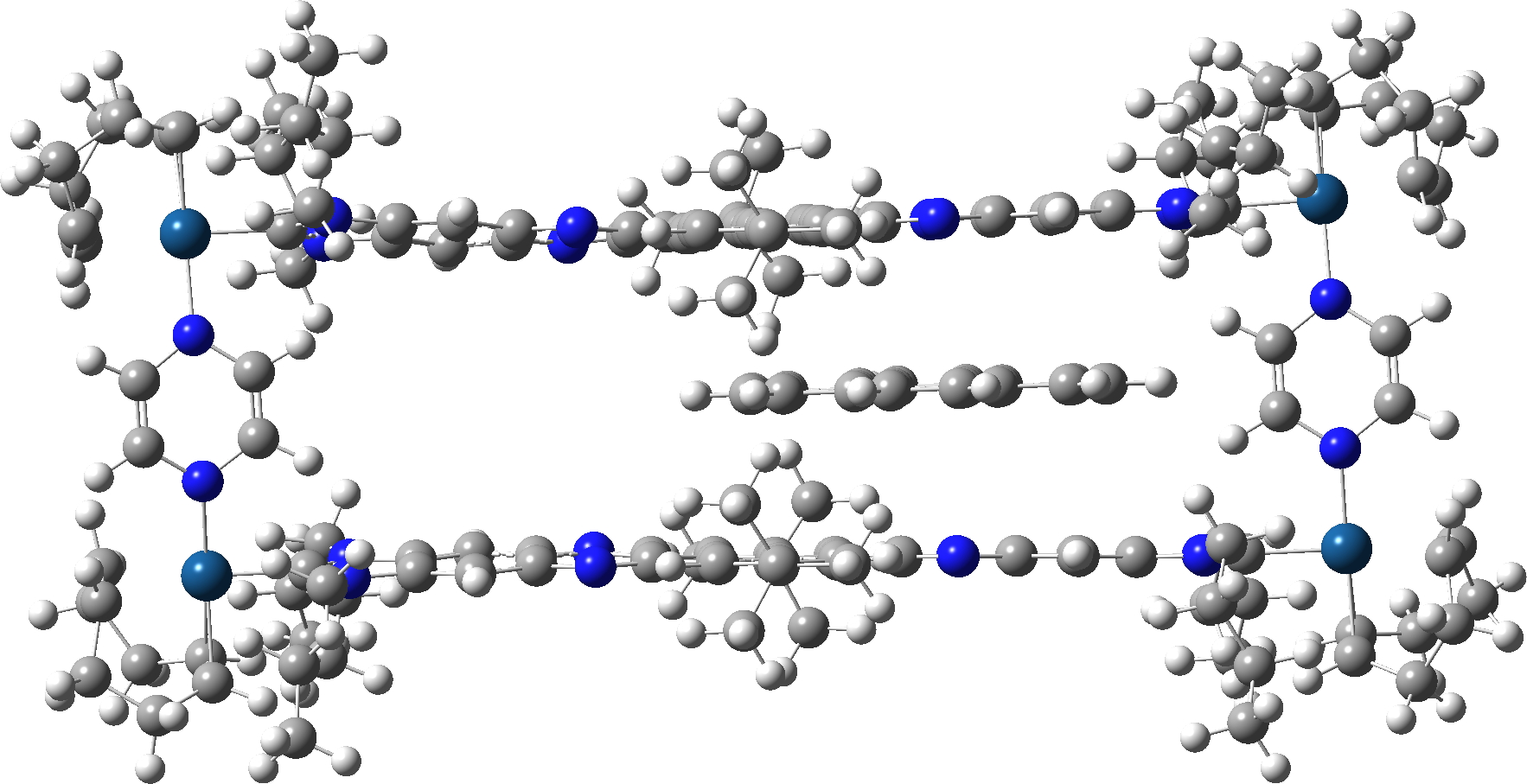
A spacefill representation shows the coronene is a perfect fit inside the cavity!
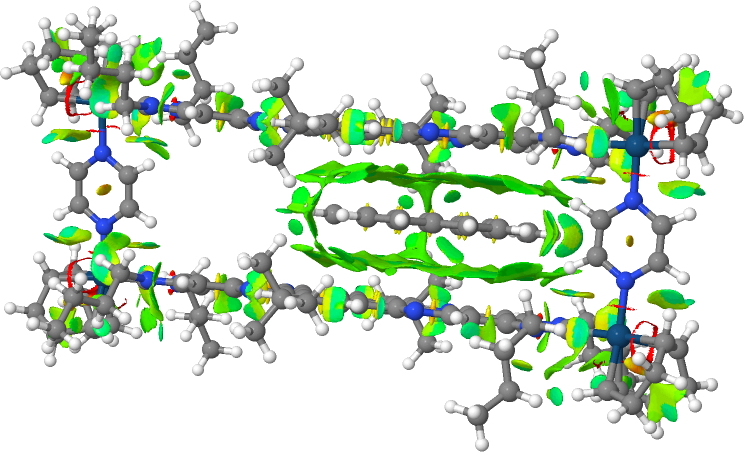
An NCI analysis (non-covalent-interaction) shows the NCI region around the coronene providing the dispersion stabilisation of the complex. The red regions by the way are related to the Ir, which has very different NCI cut-offs compared to C,N,O and shows up as an artefact.
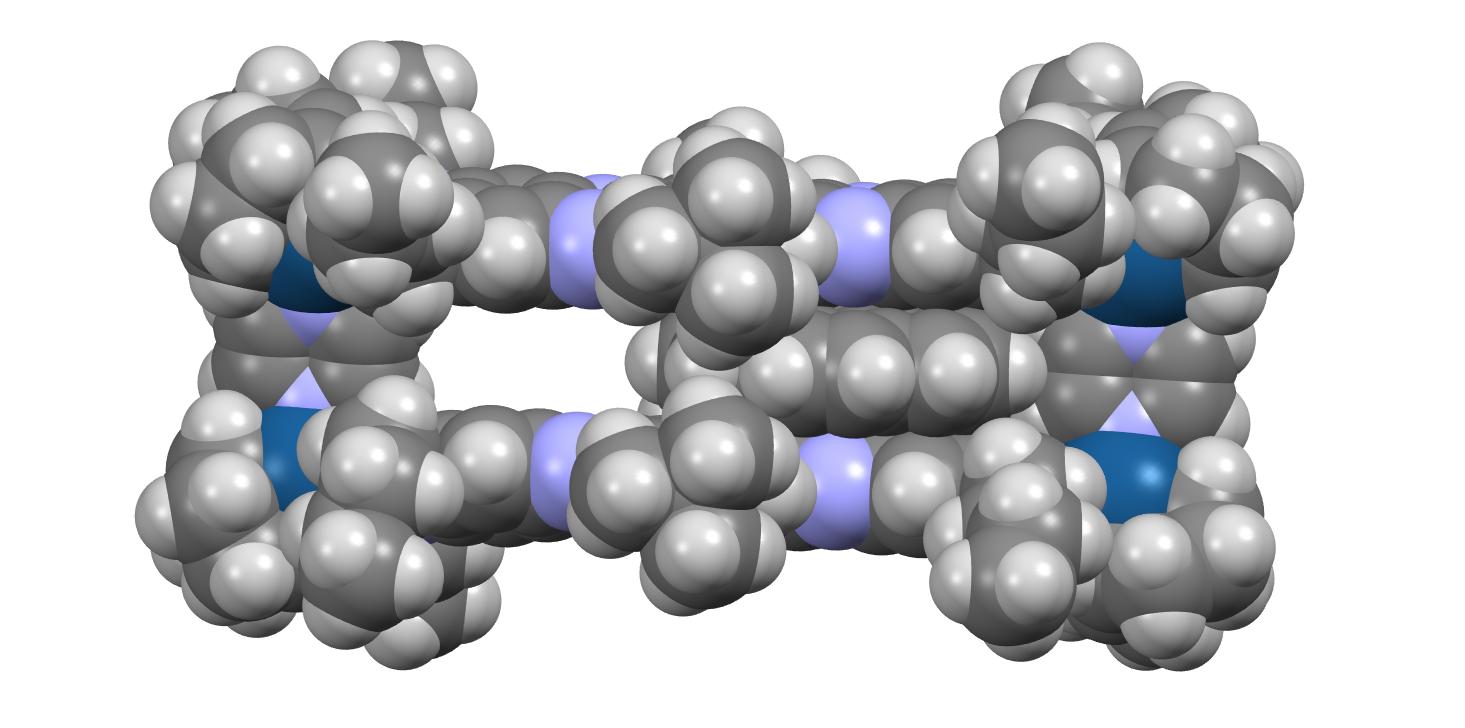
The barrier is induced by steric interactions between the coronene and the t-butyl groups attached to the edge of the cavitand, shown with a red arrow in the spacefill representation below.
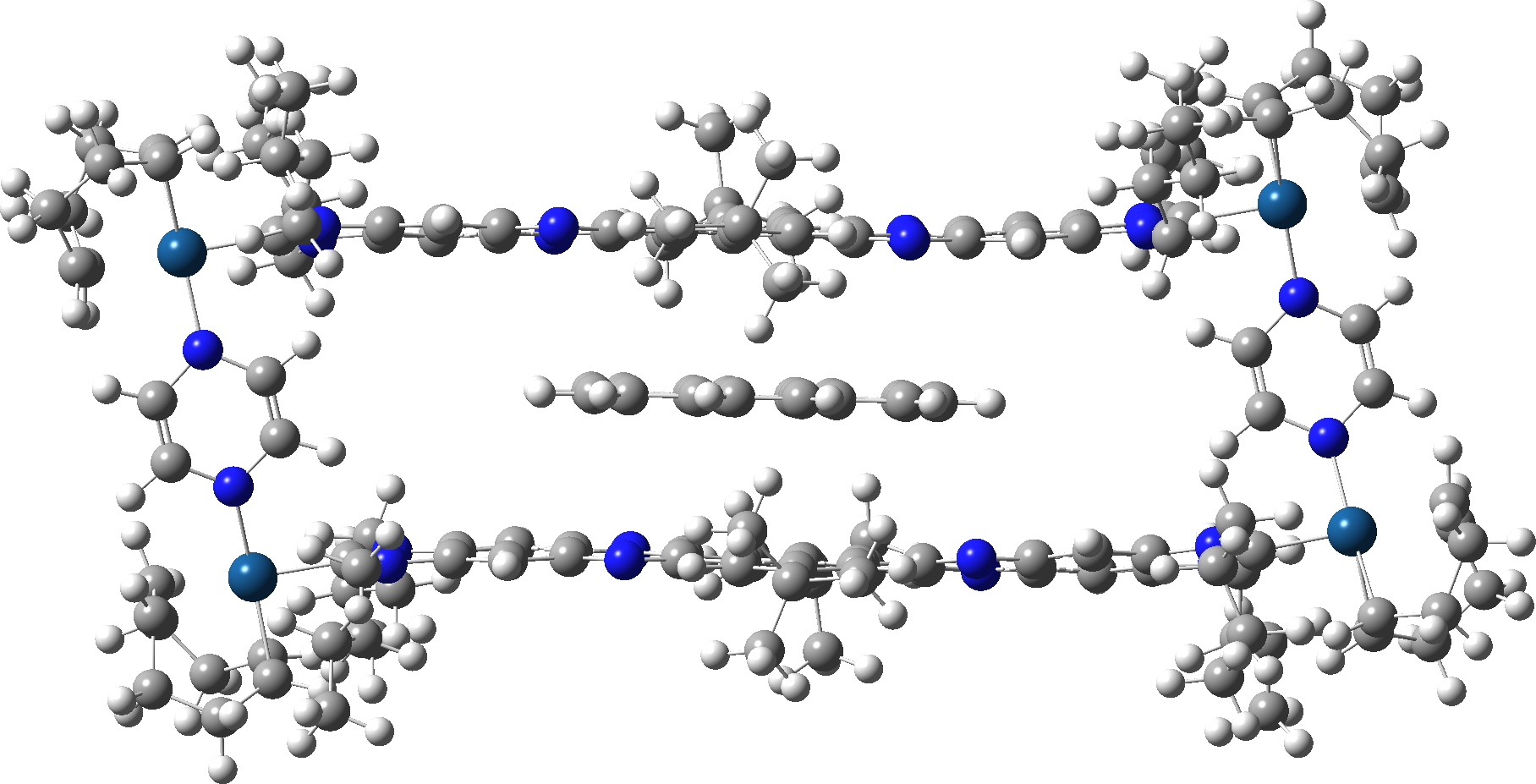
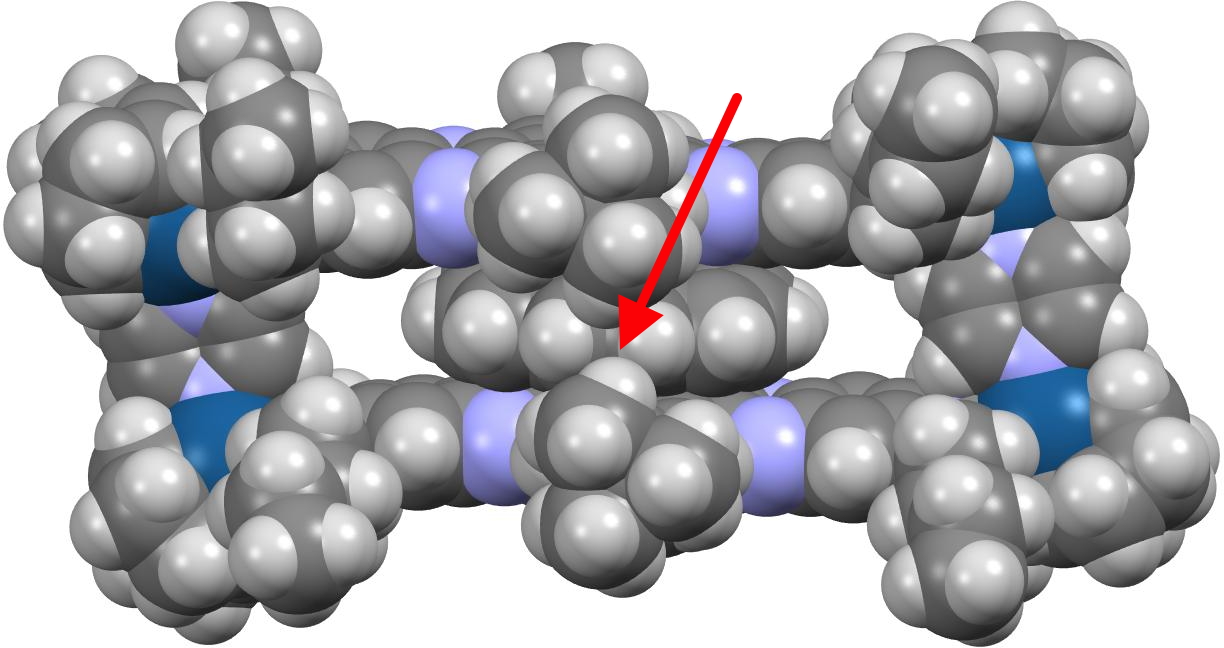
Here is the crunch, the calculated ωB97XD/Def2-SVP barrier is ΔG‡ 5.7 kcal/mol, significantly less than the value of ~13 kcal/mol measured for this dynamic process. But wait, another intermediate was located, shown below, now only 3.8 kcal/mol above the structure shown above. So the energy potential inside the cavity is more complex than just two minima and one transition state!
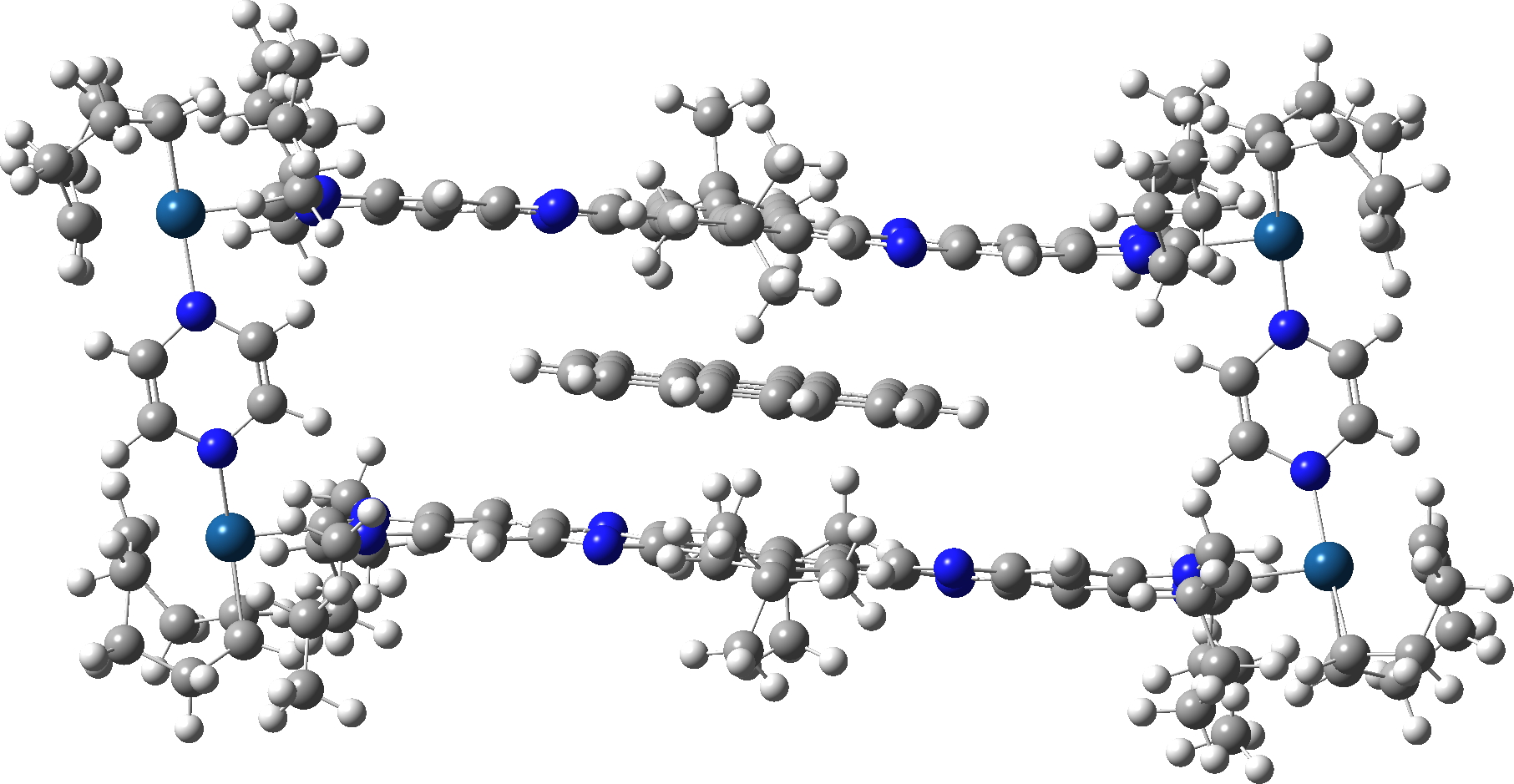
What are we to make of the disparity between the measured NMR barrier for the shuttle to move from one end of the cavitand to the other and the calculated value? Well, the barrier is likely to mostly arise from dispersion interactions, thus making this molecule a very sensitive test of how accurately the dispersion interactions are being calculated. It is known that the ωB97XD method does rather over-estimate these, and perhaps this is resulting in a barrier which is considerably too low? So this makes this molecule a useful test of potentially more-accurate dispersion corrected methods! The B3LYP+GD3+BJ method for this barrier is 6.6 kcal/mol [10],[11]. When new dispersion methods become available, I might add these as well to see if a trend develops.
References
- S. Ibáñez, P. Salvà, L.N. Dawe, and E. Peris, "Guest‐Shuttling in a Nanosized Metallobox", Angewandte Chemie International Edition, vol. 63, 2024. https://doi.org/10.1002/anie.202318829
- Y. Wu, S. Aslani, H. Han, C. Tang, G. Wu, X. Li, H. Wu, C.L. Stern, Q. Guo, Y. Qiu, A.X. Chen, Y. Jiao, R. Zhang, A.H.G. David, D.W. Armstrong, and J. Fraser Stoddart, "Mirror-image cyclodextrins", Nature Synthesis, vol. 3, pp. 698-706, 2024. https://doi.org/10.1038/s44160-024-00495-8
- L. McDermott, Z.G. Walters, S.A. French, A.M. Clark, J. Ding, A.V. Kelleghan, K.N. Houk, and N.K. Garg, "A solution to the anti-Bredt olefin synthesis problem", Science, vol. 386, 2024. https://doi.org/10.1126/science.adq3519
- D.M. Driscoll, F.D. White, S. Pramanik, J.D. Einkauf, B. Ravel, D. Bykov, S. Roy, R.T. Mayes, L.H. Delmau, S.K. Cary, T. Dyke, A. Miller, M. Silveira, S.M. VanCleve, S.M. Davern, S. Jansone-Popova, I. Popovs, and A.S. Ivanov, "Observation of a promethium complex in solution", Nature, vol. 629, pp. 819-823, 2024. https://doi.org/10.1038/s41586-024-07267-6
- T. Shimajiri, S. Kawaguchi, T. Suzuki, and Y. Ishigaki, "Direct evidence for a carbon–carbon one-electron σ-bond", Nature, vol. 634, pp. 347-351, 2024. https://doi.org/10.1038/s41586-024-07965-1
- R.C. Rohde, K.M. Carsch, M.N. Dods, H.Z.H. Jiang, A.R. McIsaac, R.A. Klein, H. Kwon, S.L. Karstens, Y. Wang, A.J. Huang, J.W. Taylor, Y. Yabuuchi, N.V. Tkachenko, K.R. Meihaus, H. Furukawa, D.R. Yahne, K.E. Engler, K.C. Bustillo, A.M. Minor, J.A. Reimer, M. Head-Gordon, C.M. Brown, and J.R. Long, "High-temperature carbon dioxide capture in a porous material with terminal zinc hydride sites", Science, vol. 386, pp. 814-819, 2024. https://doi.org/10.1126/science.adk5697
- H. Rzepa, "Molecular shuttle, wB97XD/Def2-svp, G = -8172.276752", 2025. https://doi.org/10.5281/zenodo.14746877
- H. Rzepa, "Molecular shuttle, wB97XD/def2-svp TS half way point, E = -8175.3727 G = -8172.267664 DG = 5.7", 2025. https://doi.org/10.5281/zenodo.14746910
- H. Rzepa, "Molecular shuttle, wB97XD/def2-svp TS half way point, (calcfc) optimisation G =-8172.270606 DG = 3.8", 2025. https://doi.org/10.5281/zenodo.14746936
- H. Rzepa, "Molecular shuttle, B3LYP+GD3+BJ/Def2-svp, G = -8175.526700", 2025. https://doi.org/10.5281/zenodo.14748031
- H. Rzepa, "Molecular shuttle, B3LYP+GD3+BJ/def2-svp TS half way point, -8175.516132 DG = 6.6", 2025. https://doi.org/10.5281/zenodo.14748035