Much like chocolate, some of us metallaholics cannot get enough. So WUQXIP proved an irresistible frolic (DOI: 10.1021/om020789h). Let us start with benzene. It can have metals added in two ways, whilst preserving its essential aromaticity.
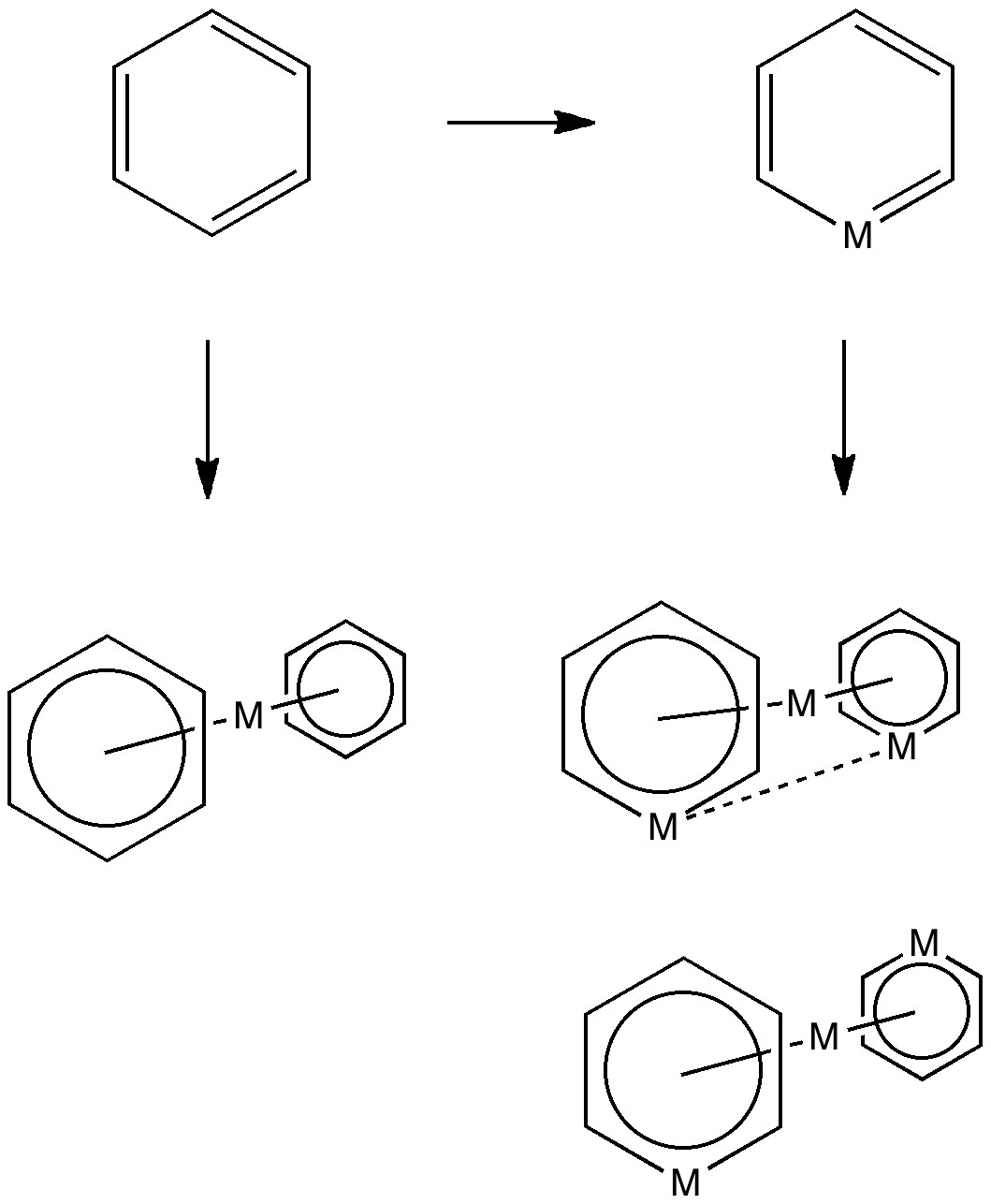
Triple metal delight.
Making a metal sandwich is of course very well known, ferrocene being the first example where the bonding was identified. Replacing a carbon with a metal is a little more recent, having been suggested by Hoffmann in 1979. This is known as a metallabenzene. The compound below combines both features.
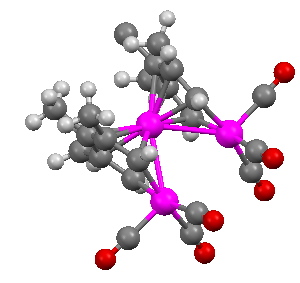
WUQXIP. Click for 3D
The metal is Ruthenium (coloured purple above) in the same column of the periodic table as Iron. What of the triple delight? Well, the compound really is more than the sum of its parts. The original authors (Ulrike Effertz, Ulli Englert, Frank Podewils, Albrecht Salzer, Trixie Wagner and Martin Kaupp) speculated about whether there might be bonding between any of the Ru atoms. You see an apparent mutual attraction meant that they were rather closer than the sum of their van der Waals radii would suggest they should be. Their relative orientation (they could easily have avoided each other) was very suggestive. So a metal-metal bond would be our third delight! To find out, the authors noted above did an ELF analysis. I have repeated this and the result is shown below.
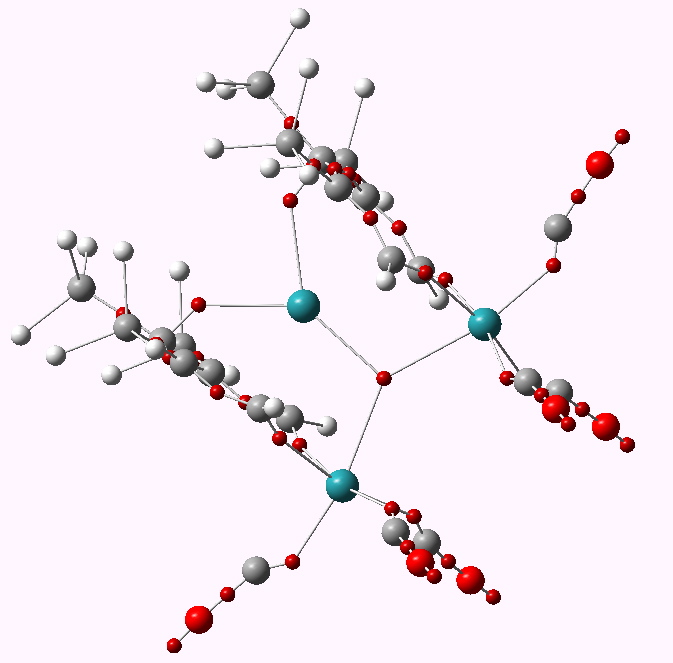
ELF basins for WUQXIP. Click for 3D
A
trisynaptic basin is revealed between the three ruthenium atoms; a classic example of a three-centre bond. It integrates to 0.64 electrons (
B3PW91/Dev-2-pVDZ), so its fairly weak. But still strong enough to bring the three ruthenium atoms closer together. A true triple metal bonding delight!
-
Henry Rzepa is Emeritus Professor of Computational Chemistry at Imperial College London.
View all posts
Related
Tags: Albrecht Salzer, Frank Podewils, Martin Kaupp, metal, metal delight, metal sandwich, metal-metal bond, metallabenzene, metallocene, three-centre bonding, triple metal bonding delight, Triple metal delight, Trixie Wagner, Ulli Englert, Ulrike Effertz
This entry was posted on Tuesday, March 1st, 2011 at 7:46 pm and is filed under Hypervalency. You can follow any responses to this entry through the RSS 2.0 feed.
You can leave a response, or trackback from your own site.


