A little while ago, I speculated (blogs are good for that sort of thing) about hexavalent carbon, and noted how one often needs to make (retrospectively) obvious connections between two different areas of chemistry. That post has attracted a number of comments in the two years its been up, along the lines: what about carboranes? So here I have decided to explore that portal into boron chemistry. The starting point is the reported crystal structure of a molecule containing a CH12B11– anion (DOI: 10.1021/ja00201a073). This differs from the molecule I previously reported in having not so much 5C-C + 1C-H bonds around a single carbon, but instead 5B-C + 1C-H bonds. The basic cluster is much in fashion (as B12Cl122-) for its properties as a non-coordinating counterion.
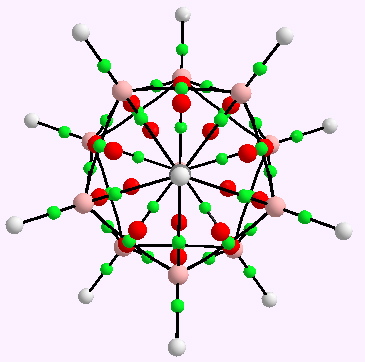
The CH12B11 (-) anion. AIM analysis. Click for 3D.
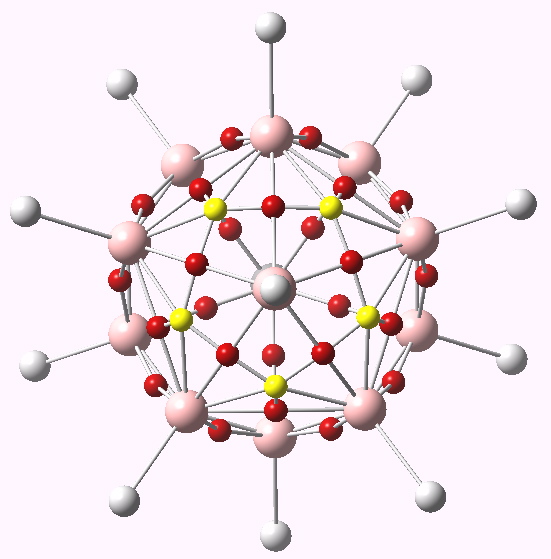
The CH12B11 (-) anion ELF basins. Click for 3D.
This molecule reveals quite clearly how sterile the debate is about whether carbon can be hypervalent. If the Lewis definition of a bond as an electron pair is removed, then hypervalency exceeding four can easily be obtained. What is certainly sacrosanct is the valence shell octet for carbon (and boron).
Finally, I throw B12H122- in. This has the wonderful icosahedral symmetry, and as you might expect, each boron is defined by five two-centre B-B bonds (~0.66e) and five three-centre BBB bonds (~0.24e), as well as a conventional two-electron B-H bond. Each boron is undecavalent! Not seen that particular valency before!!
My closing remark above was that I had not seen the term undecavalent used before. Indeed, it never seems to have been (in CAS). But I did come across many uses of the term decavalent. It seems that proteins can bind decavalently! Quite a departure from the normal meaning of the term. In ontological senses, one has to define one’s schema!