
2,4,6-trinitrotoluene is better known by its initials, TNT. It is an important explosive, since it can very quickly change from a solid into hot expanding gases. Two moles of solid TNT almost instantly changes to 15 moles of hot gases plus some powdered carbon, which gives a dark sooty appearance to the explosion. This is where another explosive, nitroglycerin, has an advantage, since it produces a smokeless explosion, allowing artillery and naval gunners to fire without their field of vision becoming obscured by smoke during the action.
TNT is made by adding 3 NO2 groups (from nitric acid) to toluene. At low temperatures, the mononitrotoluenes are made, and as the reaction temperature is increased the dinitro compounds are formed, until eventually TNT is formed.

 TNT is explosive for two reasons. First, it contains the elements carbon, oxygen and nitrogen, which means that when the material burns it produces highly stable substances (CO, CO2 and N2) with strong bonds, so releasing a great deal of energy. This is a common feature of most explosives; they invariably consist of many nitrogen or oxygen containing groups (usually in the form of 2, 3 or more nitro-groups), attached to a small, constricted organic backbone.
TNT is explosive for two reasons. First, it contains the elements carbon, oxygen and nitrogen, which means that when the material burns it produces highly stable substances (CO, CO2 and N2) with strong bonds, so releasing a great deal of energy. This is a common feature of most explosives; they invariably consist of many nitrogen or oxygen containing groups (usually in the form of 2, 3 or more nitro-groups), attached to a small, constricted organic backbone.
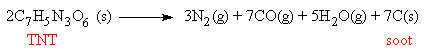
However, explosives like TNT, actually have less potential energy than gasoline, but it is the high velocity at which this energy is released that produces the blast pressure. This very high speed reaction is called a detonation. TNT has a detonation velocity of 6,940 m/s compared to 1,680 m/s for the detonation of pentane in air, and the 0.34 m/s stoichiometric flame speed of gasoline combustion in air.
 The second fact that makes TNT explosive is that it is chemically unstable - the nitro groups are so closely packed that they experience a great deal of strain and hindrance to movement from their neighbouring groups. Thus it doesn't take much of an initiating force to break some of the strained bonds, and the molecule then flies apart. Typically 1 gram of TNT produces about 1 litre of gas, which is a 1000 fold increase in volume. This expanding hot gas can be used to propel a projectile, such as a bullet from a gun, or for demolition purposes.
The second fact that makes TNT explosive is that it is chemically unstable - the nitro groups are so closely packed that they experience a great deal of strain and hindrance to movement from their neighbouring groups. Thus it doesn't take much of an initiating force to break some of the strained bonds, and the molecule then flies apart. Typically 1 gram of TNT produces about 1 litre of gas, which is a 1000 fold increase in volume. This expanding hot gas can be used to propel a projectile, such as a bullet from a gun, or for demolition purposes.
 There are a number of advantages that TNT has for ammunition manufacturers. First, it melts at a reasonably low temperature (81°C), which means it can be readily melted and poured into shells and bombs. Secondly, it is not too unstable - allowing it to be handled reasonably safely during manufacture and operation. TNT will not spontaneously explode, and in fact can be treated quite roughly. In order to initiate the explosion, TNT must first be detonated using a pressure wave from another, more easily induced explosion from another explosive called a detonator. One such detonator is lead azide, Pb(N3)2, which explodes when struck or if an electric discharge is passed through it.
There are a number of advantages that TNT has for ammunition manufacturers. First, it melts at a reasonably low temperature (81°C), which means it can be readily melted and poured into shells and bombs. Secondly, it is not too unstable - allowing it to be handled reasonably safely during manufacture and operation. TNT will not spontaneously explode, and in fact can be treated quite roughly. In order to initiate the explosion, TNT must first be detonated using a pressure wave from another, more easily induced explosion from another explosive called a detonator. One such detonator is lead azide, Pb(N3)2, which explodes when struck or if an electric discharge is passed through it.
Plastic explosives have been around for a couple of decades. They typically consist of an explosive mixed with an oil or wax plastic resin. One example is C4 (plasticized RDX), which has been used by the military (and safecrackers!) for years. RDX is cyclotrimethylenetrinitramine, another high explosive with a tight, constricted ring structure similar to TNT except containing even more energy-providing N atoms. Recently, however, a range of plastic explosives have been produced by the Semtin Glassworks in Czechoslovakia (now known as VCHZ Synthesia), and were christened Semtex. Semtex is extremely powerful for its weight, and is harder to detect than other explosives because it has little smell. As a result, it has become the explosive of choice by various terrorist groups around the world. Although examples are of variable composition, it typically contains approximately 8% oil, 9% rubber, and approximately equal quantities of RDX and pentaerythritol tetranitrate (PETN).
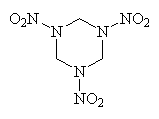 |  |
| RDX | PETN |