Mod:phys3
From ChemWiki
See also: Windows mode, Linux mode, Programs, Module 1, Module 2, Module 3
Contents[hide] |
[edit] Module 3, Experiment 3
[edit] Exercise
Use the the AM1 semi-empirical molecular orbital method for these calculations (to start with).
i) Use GaussView to build cis butadiene, and optimize the geometry using Gaussian. Plot the HOMO and LUMO of cis butadiene and determine its symmetry (symmetric or anti-symmetric) with respect to plane.
There are two ways to do this in GaussView. One is: Select Edit→MOs. Select the HOMO and the LUMO from the MO list (highlights it yellow). Click the button Visualise (not Calculation), then Update. Alternately, having calculated the surface for this orbital, you can display it in the main GaussView window for the molecule, from the Results→Surfaces menu. Select Surface Actions→Show Surface. Having displayed the surface this way, you can also select View→Display Format→Surface, and change Solid to Mesh.
ii) Computation of the Transition State geometry for the prototype reaction and an examination of the nature of the reaction path.
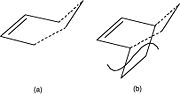
The transition structure has an envelope type structure, which maximizes the overlap between the ethylene π orbitals and the π system of butadiene. One way to obtain the starting geometry is to build the bicyclo system (b) and then remove the -CH2-CH2- fragment. One must then guess the interfragment distance (dashed lines) and optimize the structure, but use any method you wish, based on the tutorial above, to characterise the transition structure. Confirm you have obtained a transition structure for the Diels Alder reaction!
Once you have obtained the correct structure, plot the HOMO as in (i). Rotate the molecule so that the symmetry and nodal properties of the system can be interpreted, and save a copy of the image.
(iii) To Study the regioselectivity of the Diels Alder Reaction
Cyclohexa-1,3-diene 1 undergoes facile reaction with maleic anhydride 2 to give primarily the endo adduct. The reaction is supposed to be kinetically controlled so that the exo transition state should be higher in energy.
Locate the transition structures for both 3 and 4. Compare the energies of the endo and exo forms.
Measure the bond lengths of the partly formed σ C-C bonds and the other C-C distances. Make a sketch with the important bond lengths. Measure the orientation, (C-C through space distances between the C=O-CO-C=O- fragment of the maleic anhydride and the C atoms of the “opposite” -CH2-CH2- for the exo and the “opposite” -CH=CH- for the endo). The structure must be a compromise between steric repulsions of the -CH2-CH2- fragment and the maleic anhydride for the exo versus secondary orbital interactions between the π systems of -CH=CH- and -C=O-CO-C=O- fragment for the endo.
Plot the HOMO as in the previous exercise. Examine carefully the nodal properties of the HOMO between the -C=O-CO-C=O- fragment and the remainder of the system. What can you conclude about the so called “secondary orbital overlap effect”?
See also: Windows mode, Linux mode, Programs, Module 1, Module 2, Module 3
© 2008 Imperial College London