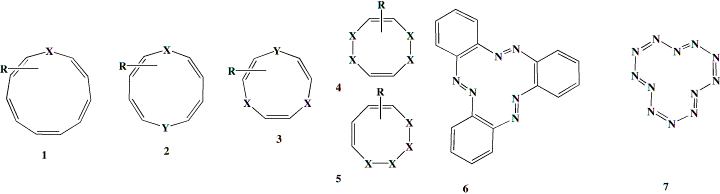
Summary: Conformations of the mono, di, tri and tetra hetero [12] annulenes which have C2 or pseudo C2 symmetry are explored using ab initio theory for their potential Möbius aromatic properties, as quantified using the nucleus independent chemical shift (NICS) values at the ring centroid. This is found to be greatest for the sulphur heterocycles, and a number of known examples of tri and tetrahetero systems show C2 symmetric conformations for the crystal structures and modestly aromatic NICS values. The structures of several other annulenes show distorsions resulting from Möbius aromaticity, most promimently the tetrathiocine and tetraselenocine ring systems. We also propose forms of the elements nitrogen and sulfur which have some Möbius-aromatic character, the latter in particular being only 11 kcal mol-1 higher than the most stable conformation.

A search of the Cambridge crystallographic database8 reveals several carbocyclic annulene structures which have not hitherto been analysed in the context of Möbius aromaticity.9 Thus crystal structures of derivatives of [12]10, [16], [20] and [24]11 annulenes have been reported. The [20] annulene 4 (a=2,b=1) reveals D2 symmetry whilst the lower homologue [12] annulene 5 (R=CC-tBu) shows a very small degree of C2 twisting, which could indicate some Möbius character or could be due to crystal packing.12 The [24] annulene has D2d symmetry and the biphenyl-derived [12] annulene10 4 (a=1,b=0) has the lower (and chiral) D2 symmetry. Although structures for other [12] annulenes appear in this database, they are constrained by e.g. methano bridges and hence cannot adopt conformations containing axes of symmetry.
The hetero[12]annulene series reveals several entries for compounds of the general type 1-3. Thus structure searches revealed tribenzo derivatives of 1, X=Y=O13 and X=Y=S.13 These both exhibit a formal C2 symmetry axis. One example containing X=S, Y=N-acetyl15 is identified as having a pseudo C2 symmetry axis which passes through one S and the opposing C=C bond. These species can all be described as having conformations formed by replacing three C=C units in the recently identified Möbius form of [12] annulene5 with the corresponding heteroatom. No examples of the ring system 2 were found and only a single example of a methano-bridged derivative of the [12] heteroannulene 3, X=S has been reported,14 which by its nature has an enforced plane rather than axis of symmetry.
There are also several interesting heteroatom rich systems worthy of comment. The unusual hexa-aza[12] annulene 616 has distinct C2 like distorsions from planarity. Six examples of the 12p ring system 7a (X=S, tetrathiocin) are known17 to exhibit C2 ring symmetry, although examples of this ring with Cs symmetry are more common. The only known selenium analog 7 (X=Se, tetraselenocine) exhibits C2 ring symmetry,18 as do all the 4,5,6,7 isomers of tetrathiocin (7b, X=S) which are also not rare.19 Both AgS9-1 and AuS9-1 salts 8 are known20 in which the 10-membered ring shows C2 symmetry, and which could be formally at least be regarded as a [20] annulenes.
We conclude that a search of the Cambridge database does seem to reveal tantalising evidence of stable Möbius conformations of various annulenes, and the examples we have cited above might represent only a small proportion of the total. To understand how the geometrical preferences of these systems arise, we turned to ab initio modelling at the B3LYP/6-31G(d) theoretical level.
| 7,X=S,R=F: HOMO-1 | 7,X=S,R=F: HOMO-3 |
|---|---|
 |
 |
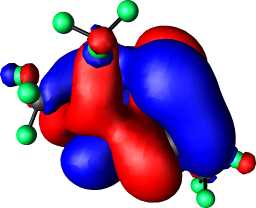
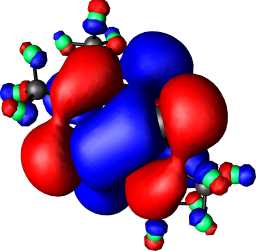
| 7,X=Se,R=CF3: HOMO | 7,X=Se,R=CF3 HOMO-1 |
 |
 |
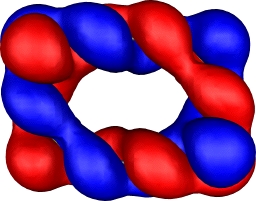
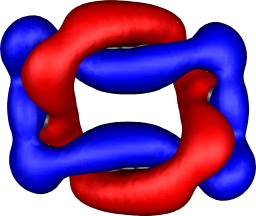
| 9,a=2,b=1: HOMO | 9,a=2,b=1: HOMO-9 |
 |
 |
| 10: HOMO-1 | 10: HOMO-2 |
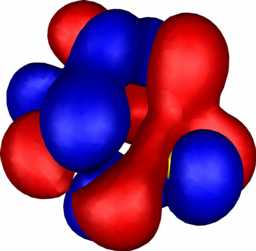 |
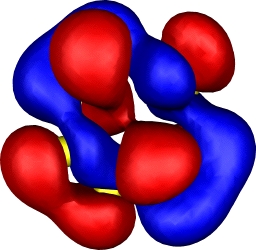 |
| 10: HOMO-4 | 10: HOMO-7 |
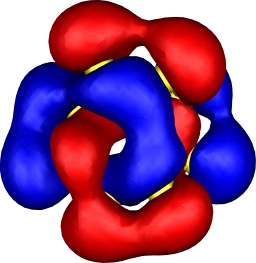 |
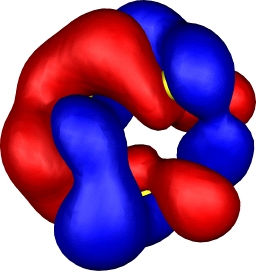 |
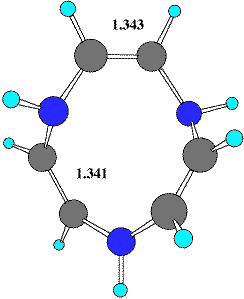
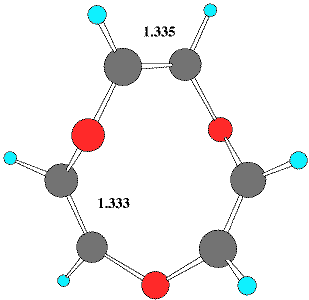
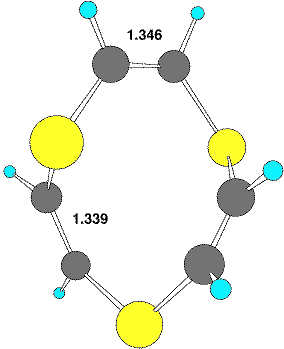
| Table 1. Calculated energies (B3LYP/6-31G(d), Hartree) and NICS(0) values (ppm) for 4-6 | |||
|---|---|---|---|
| System | Energy | NICS(0) | |
| 4,a=1,b=0 | -1076.47089 | -3.0 | |
| 4,a=1,b=1 | -1228.84187 | 1.4 |
|
| 4,a=2,b=1 | -1381.16171 | 0.5 | |
| 4,a=2,b=2 | -1533.47962 | 0.6 |
|
| 5 | -766.71065 | 5.7 |
|
| Table 2. Calculated energies (B3LYP/6-31G(d), Hartree) or relative energies(kcal/mol) forheteroannulenes 1-3, 7, 8, and NICS(0) values (ppm). | ||||
|---|---|---|---|---|
| X, Y, R | Cs (C1) | C2 (pseudo C2) | NICS(0) | |
| 1, X=Y=NH, R=H | 13.5 (41.6) | 2.5, 3.1 (-398.20690) | 1.3 | |
| 1, X=Y=O, R=H | 6.4, 46.3 | -457.78250 | 1.5 |
|
| 1, X=Y=O, R=F | -3.1, 0.1, 38.3 | -1053.15767 | -2.3 | |
| 1, X=Y=PH, R=H | 4.3 | (-1258.00411) | 0.1 |
|
| 1, X=Y=S, R=H | 6.7, 18.7 | -1426.72236 | 0.0 |
|
| 1, X=Y=S, R=F | -5.1, 12.7 | -2022.06902 | -3.1 | |
| 1, X=O, Y=CH-, R=H | -3.2 | -421.26886 | 2.8 |
|
| 1, X=O, Y=CF-, R=F | 1.7 | -1115.91871 | -1.0 | |
| 1, X=O, Y=S, R=H | 5.6, 29.2 | -780.76745 (2.0) | 0.7 |
|
| 1, X=O, Y=S, R=F | 28.2 | (-1376.13160) | -2.8 | |
| 1, X=S, Y=CH-, R=H | 9.1,14.1 | -1067.25508 | 5.3 |
|
| 1, X=S, Y=CF-, R=F | 4.4, 47.2 | -1761.88127 | -0.2 | |
| 1, X=S, Y=NH, R=H | -3.2 , (40.9), 24.8 | ( -1083.88695) | 0.2 | |
| 1, X=S, Y=O, R=H | 26.4 | -1103.74840 (1.6) | 0.0 |
|
| 1, X=S, Y=O, R=F | -3.8, 1.8,29.7 | -1699.10275 | -3.3 | |
| 2, X=Y=N | -11.3 | 5.3, -420.27282 | -2.9, -1.2 | |
| 2, X=Y=O | -2.8 | 8.4, -459.99477 | -0.1, -0.6 | |
| 2, X=Y=S | 3.2, 16.8 | 1.8, -1105.94349 | -4.8, -1.4 | |
| 2, X=Y=S, R=F | -10.2 | -1899.74067 | -4.6 | |
| 3, X=NH, R=H | -8.8, 6.3 (-12.0) | -442.31797 | -5.4 | |
| 3, X=O, R=H | -1.5, 3.5 (-7.4) | -462.17742 | -5.6 | |
| 3, X=S, R=H | 1.2,8.2,(-4.0) | -785.15962 | -6.3 | |
| 3, X=S, R=F | -1.1 (-3.1) | -1777.42173 | -4.8 | |
| 6 | - | (-1021.50351) | -5.9 | |
| 7a, X=S, R=H | -1747.52621 | -1747.53529 | -4.7 | |
| 7b, X=S, R=H | - | -1747.55077 | -3.3 | |
| 7a, X=S, R=F | -2144.43257 | -2144.42289 | -3.9 | |
| 7a, X=Se, R=H | -9752.33901 | -9752.34808 | -6.2 | |
| 7b, X=Se, R=H | (-9752.37322) | -9752.37739 | -1.6 | |
| 7a, X=Se, R=F | -10149.251472 | -10149.24279 | -4.9 | |
| 7a, X=Se, R=CF3 | - | -11100.4724 | -7.6 | |
| 8,X=Ag | - | -8743.56809b | -4.2 | |
| 9,a=1,b=0 | - | -1745.01525 | -2.8 | |
| 9,a=2,b=1 | - | -2049.58532 | -1.1 | |
| 10 | -11.0 | -3185.53301 | -3.2 | |
| 11 | - | (-656.58174) | -4.4 | |