The new methodology referred to in this section was made possible
by two new cycloaddition reactions developed in our laboratories
at Central Queensland University (Warrener and Butler) and Deakin
University (Russell).
These are part of a suite of reactions which we have developed
as part of a building BLOCK approach to synthesis.
The concept has been to form two classes of molecular BLOCKs
corresponding to LEGO blocks. This analogy requires that the
BLOCKs are available in a range of shapes
and sizes, that they are able to be stored (stable at ambient),
and that they form rigid structures when joined. In the LEGO
block, the linking is between specific faces of the block where
recognition depends on a geometric alignment of male, female subcomponents;
the molecular equivalent uses two complementary functional groups
which react specifically with one another. Like the LEGO blocks,
similar face do not react with themselves only with partners with
appropriate parity. We have designated such blocks as A-BLOCKs
(alkenes, a-diamines etc) and B-BLOCKs
(1,3-dienes, 1,3-dipoles, a-diones).
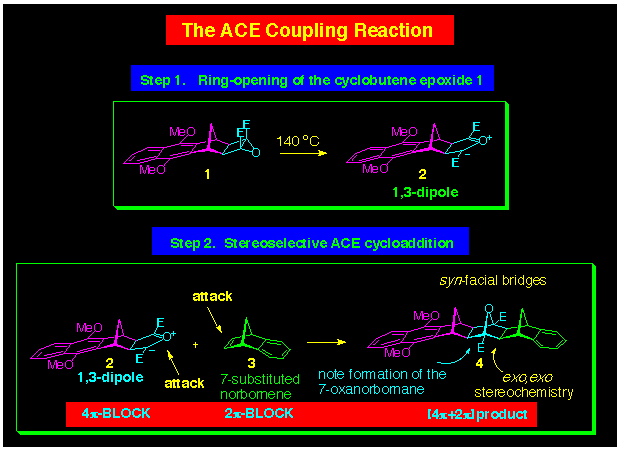
2.1 The ACE Reaction
This reaction was the first to be discovered and involves the
cycloaddition between an Alkene
and a Cyclobutene Epoxide
(ACE is an acronym for these
reagents). These two reagents react together in what is considered
to be a 1,3-dipolar reaction, although we have only indirect evidence
for this. We believe that the thermal conditions (ca 140
oC, sealed tube, DCM) generates the transient
1,3-dipole (Step 1, Scheme 1) which reacts with the alkene to
form cycloaddition products characterised by the presence of a
7oxanorbornane ring (Step 2, Scheme 1). The key feature
of this reaction is that it occurs stereoselectively when norbornenes
act as the alkene and affords a product where the norbornene methylene
bridge has syngeometry in relation to the oxygen
bridge of the newly formed 7oxanorbornane. Further, when
the cyclobutene epoxide is itself part of an alicyclic frame,
then products containing several synfacial bridges
can be produced.
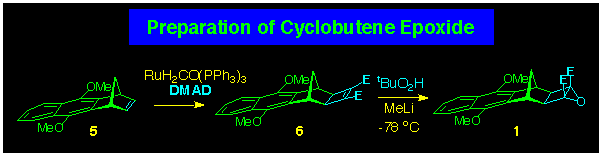
The required cyclobutene epoxides are prepared in a two-step sequence
from the corresponding norbornene. The first step is well precedented
in the original report of the ruthenium catalysed addition of
DMAD to norbornanes by Mitsudo and co-workers and has been applied
in much of our own work on molrac synthesis and functionalisation.
The epoxidation step had to be suitable for application to an
electron-deficient p-bond, and we have
found that low temperature treatment with tertiary butyl peroxide
anion achieves this goal.
A feature of the ACE reaction in BLOCK assembly
protocols is that most alkene A-BLOCKs of
the norbornene type can be converted to their B-BLOCK
counterpart, in the simple two-step protocol outlined in Scheme
2. This has special synthetic value when the A-BLOCK
is itself difficult to make, yet with little more effort can be
converted to its complementary BLOCK type.
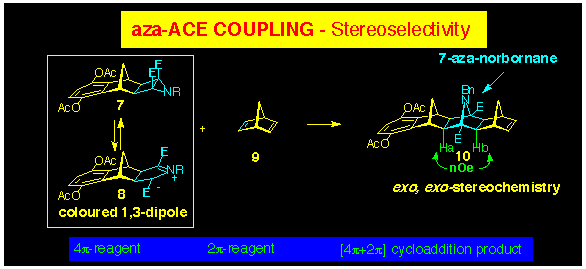
2.2 The aza-ACE
reaction
The aza-ACE reaction is the aza-analogue
of the ACE reaction where the epoxide ring has been replaced with
an aziridine. Accordingly, the products contain a 7-azanorbornane
ring and the same cycloaddition stereoselectivity is observed
on heating the aziridinocyclobutane with norbornenes as that observed
in the ACE protocol (Scheme 3). Furthermore, introduction of
heteroatoms (O or N) at the 7-position of the norbornene maintains
the same stereoselectivity and provides polynorbornane products
containing one or more 7-azabridges with syn-facial geometry
(see Section 3.2).
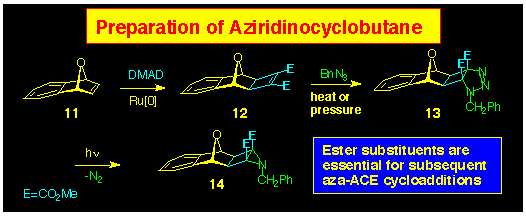
The preparation of the aziridine can be achieved in 3 steps from
the appropriate norbornene, and this is illustrated in Scheme
4 using the 7-oxa-benzonorbornene 11 as the starting product.
The initial step is common to both ACE and
aza-ACE protocols and involves formation
of the exo-fused cyclobutene 12 by ruthenium catalysed
addition of DMAD. Addition of benzylazide occurs slowly under
thermal conditions (RT, 1 week). Conversion of the triazoline
13 to the aziridine 14 occurs smoothly by irradiation
(Rayonet reactor, l 300 nm) in benzene
solution.