I noted previously that some 8-ring cyclic compounds could exist in either a planar-aromatic or a non-planar-non-aromatic mode, the mode being determined by apparently quite small changes in a ring substituent. Hunting for other examples of such chemistry on the edge, I did a search of the Cambridge crystal database for metal sulfides.
The search was specified as following:
- Any element from one of the three transition metal series, TS1, TS2 or TS3
- to contain a M-S bond (any type of bond)
- with the restriction that the sulfur has only one atom attached (the metal)
- R < 0.05
- No errors and no disorder
The results for the three transition series were quite different. The first row indicated a distribution with a single maximum (~2.3Å), albeit with a very long tail reaching out to 3.2Å. The second row had a very clear bimodel distribution, with two peaks, at ~2.2Å and ~2.55Å, the latter having the greater number of examples. The third row showed an inverse distribution, with the prominent peak at ~2.2Å and the smaller peak at 2.55Å.
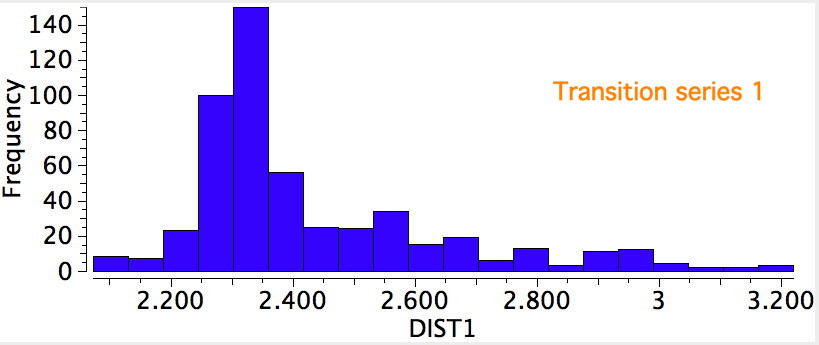
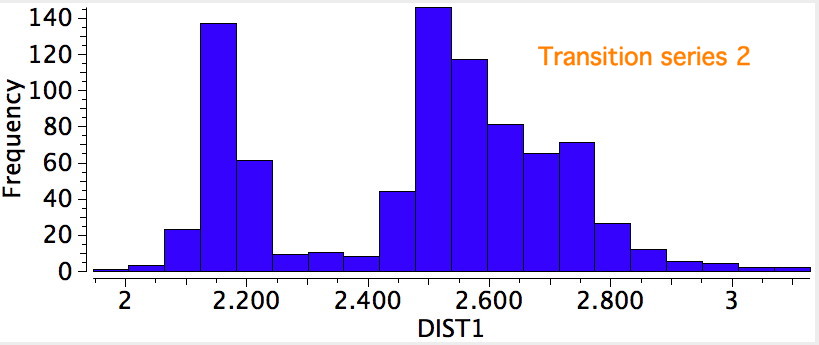
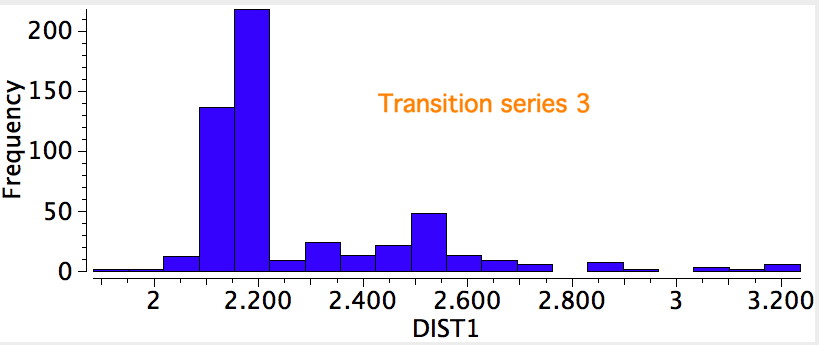
A search for the possible reasons for the bimodal distributions is now needed. This could be done in two ways; (a) to refine the search, or (b) to individually scrutinize each example to identify if any chemical reasons can be found. Since there are 100s of examples, I will concentrate on the first tactic. The most obvious is that the compounds cluster into neutral and ionic, as below, which allows the search to be constrained to only entries where a negative charge has been assigned to the sulfur.
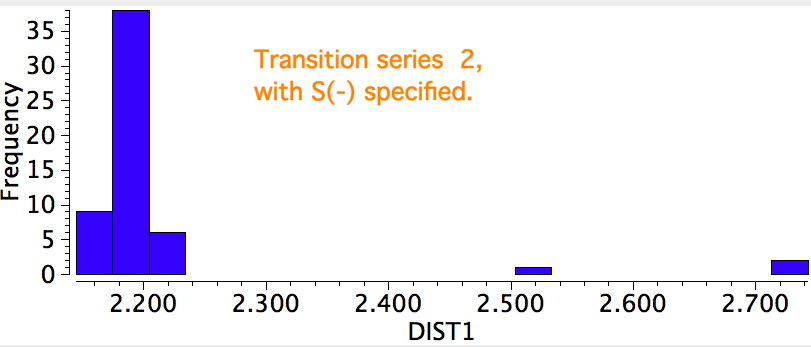
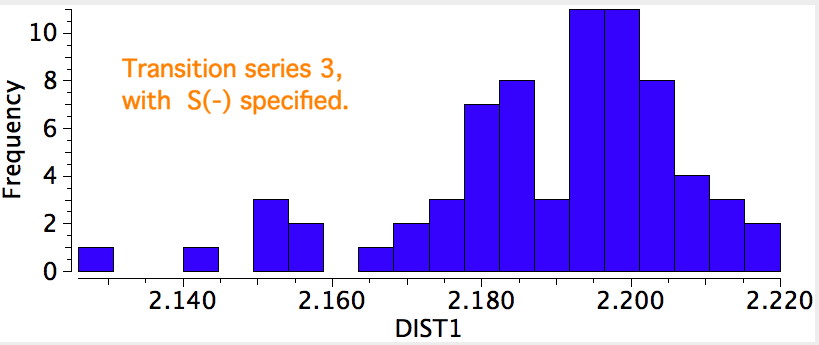
This reveals such entries are a fraction of the total, and for these the C-S bond length clusters around 2.2Å. Which leaves us with a mystery. This would mean that the neutral systems, presumably with a formal M=S bond, would have to be responsible for the entries at ~2.55Å. Could it really be that a higher bond order results in a longer bond?
What the search does not allow us to do (or I am unaware of how to do it) is to identify whether there are any pair of examples where the ligands surrounding M are identical but where one of the pair has a short and the other a long M-S bond. Or indeed such a pair where the differences between the ligands are small (however that is defined). Similarly, one cannot constrain the searches by spin state to see if that might be responsible. It would also be nice to have the system automatically evaluate whether the valence shell on the metal is full (18) or not (16, 17) to see if that is related to the bond length. Indeed, an example of needing to count the valence shell was recently brought to my attention by a student, and it will be the subject of a future post.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
Tags: Cambridge, chemical reasons, metal, metal sulfides, neutral systems, transition metal series