Not long ago, I described a cyclic carbene in which elevating the carbene lone pair into a π-system transformed it from a formally 4n-antiaromatic π-cycle into a 4n+2 aromatic π-cycle. From an entirely different area of chemistry, another example of this behaviour emerges; Schreiner’s[1] trapping and reactions of t-butyl-hydroxycarbene, as described on Steve Bachrach’s blog. A point I often make is that chemistry is all about connections, and so here I will discuss such a connection.
The essence of Schreiner’s[1] work is that once generated, t-butyl hydroxycarbene could rearrange in three different ways:
- A [1,2] hydrogen migration (blue, formally a pericyclic sigmatropic reaction)
- A [1,2] methyl migration (magenta, formally a pericyclic sigmatropic reaction)
- A C-H carbene insertion (red, formally a pericyclic cycloaddition).
As pericyclic reactions, all three would be four electron thermal processes, and again all would require an antarafacial component to be present somewhere. So I pose the question here: are they indeed true pericyclic reactions, and if so are they true four-electron ones (with an implied antarafacial component somewhere)?
I will show a computed IRC for each (ωB97XD/6-311G(d,p)). First, the [1,2] hydrogen migration.[2] This emerges as a proton transfer, with a base (the σ-carbene) abstracting a proton from an acid (the σ-O-H bond). Crucially, the C=O bond is hardly involved in the process. Put simply, the non-involvement of that C=O means the process is not pericyclic. So there is no need for an antarafacial mode.
Next, the [1,2] methyl migration (if a true 4-electron pericyclic, one might imagine it would migrate with inversion of configuration). Here, one can see two distinct phases to the migration:
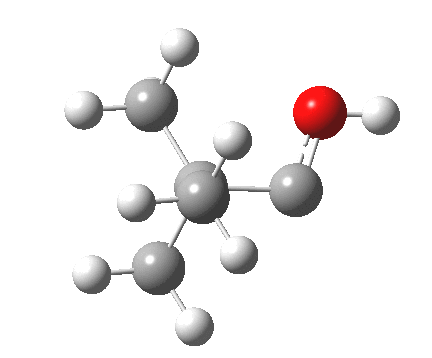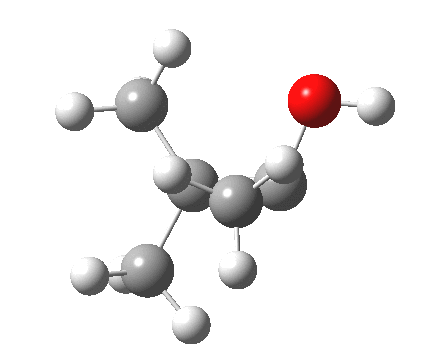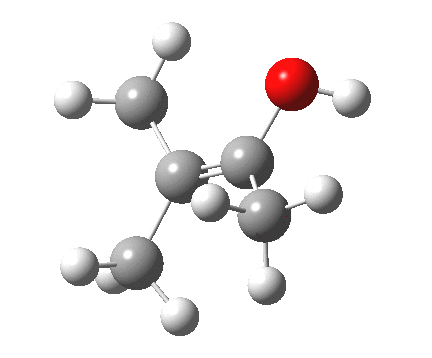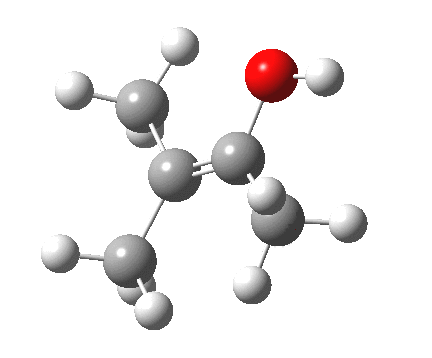
- The first phase involves a methyl migration (with retention) to form a bond by donating into the empty p-π-orbital on the carbene (the same into which two electrons were promoted to invert antiaromaticity). This is a two-electron process, analogous to a [1,2] migration in a carbocation. This phase requires no antarafacial mode.
- This forms, if you like, a zwitterionic resonance, leaving behind a carbocation, and forming a carbanion, as below.
- The second pair of electrons now come into play (the carbene pair), ending up forming the C=C bond. Crucially, this occurs AFTER the first pair have been used to migrate the methyl group. Because the sequence is now separated, this process too does not require an antarafacial mode; it effectively comprises two consecutive 2-electron processes, which overall constitute an asynchronous pericyclic process. There are no actual intermediates along the IRC (hardly a hint of even a hidden one), so it is a concerted process overall, and the zwitterionic species implied above does not actually form.
Now for the third mode, the insertion of the carbene into a C-H bond.[3] This too occurs in two phases:
.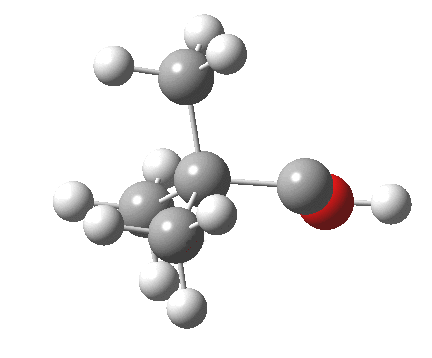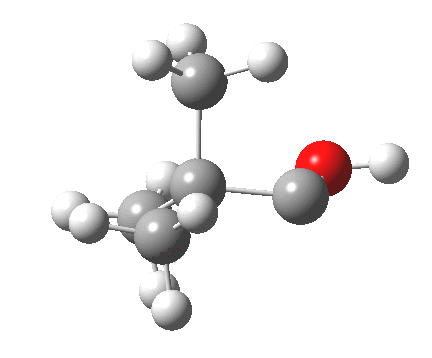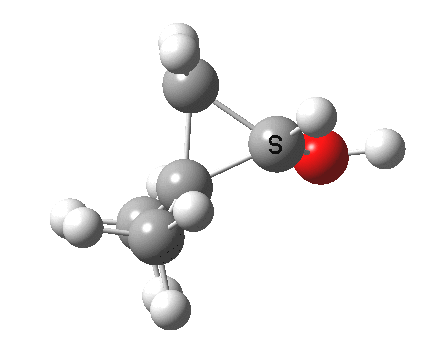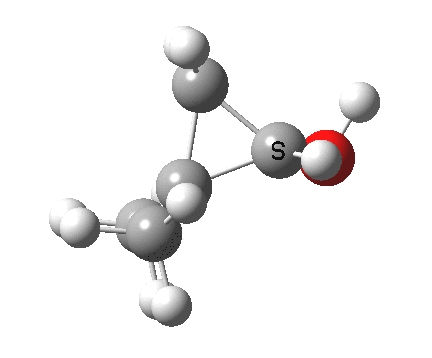
- The first phase involves the transfer of the two electrons from a C-H σ-bond into the empty p-π-orbital on the carbene (a hydride transfer).
- This forms a carbocation/carbanion zwitterionic resonance. The pyramidal carbon then inverts (umbrella mode. Is this an antarafacial mode?)
- and the carbanion then ring closes onto the carbocation to form a cyclopropane. As before, the sequence is now separated, and again does not require an antarafacial mode (?). The IRC profile (below) does appear to show a hidden intermediate (IRC = 2.9) but in fact this is the rotation of the O-H bond, and does not involve any bond formation.
So in the end, all three apparently pericyclic thermal transformations of t-butyl hydroxycarbene avoid 4-electron cyclic antiaromaticity by either becoming acyclic, or by timing the development of the two electron pairs so that they occur sequentially and not concurrently. None of the three is a true pericyclic!
References
- D. Ley, D. Gerbig, and P.R. Schreiner, "Tunneling control of chemical reactions: C–H insertion versus H-tunneling in tert-butylhydroxycarbene", Chem. Sci., vol. 4, pp. 677-684, 2013. https://doi.org/10.1039/c2sc21555a
- H.S. Rzepa, "Gaussian Job Archive for C5H10O", 2013. https://doi.org/10.6084/m9.figshare.848560
- H.S. Rzepa, "Gaussian Job Archive for C5H10O", 2013. https://doi.org/10.6084/m9.figshare.848613
Tags: Steve Bachrach