In this post, I pondered upon the C=O infra-red spectroscopic properties of esters, and showed three possible electronic influences:
The red (and blue) arrows imply the C-O bond might shorten and the C=O bond would lengthen; the green the reverse. So time for a search of the crystal structure database as a reality check. The query is as follows:
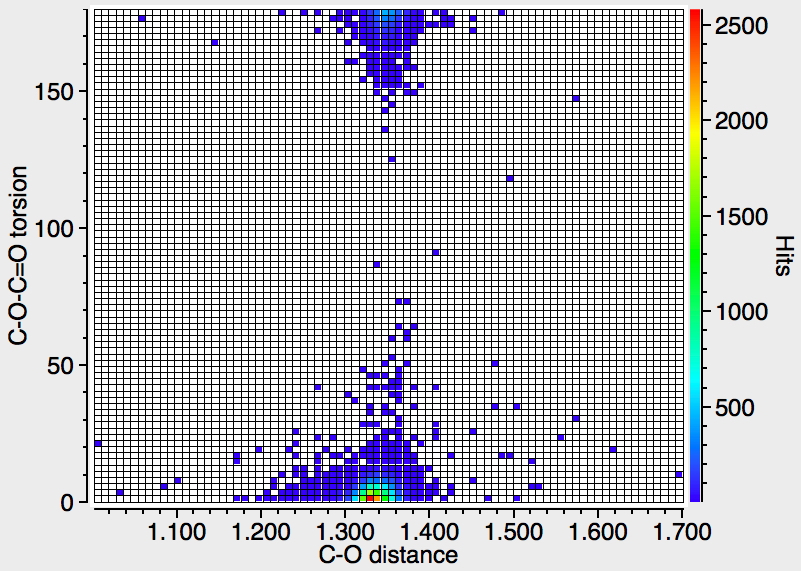
The response shows the bimodal distribution with as expected the s-cis conformation dominating. There is indeed a hint that for the s-cis, the C-O distance is rather shorter than for the s-trans conformation.
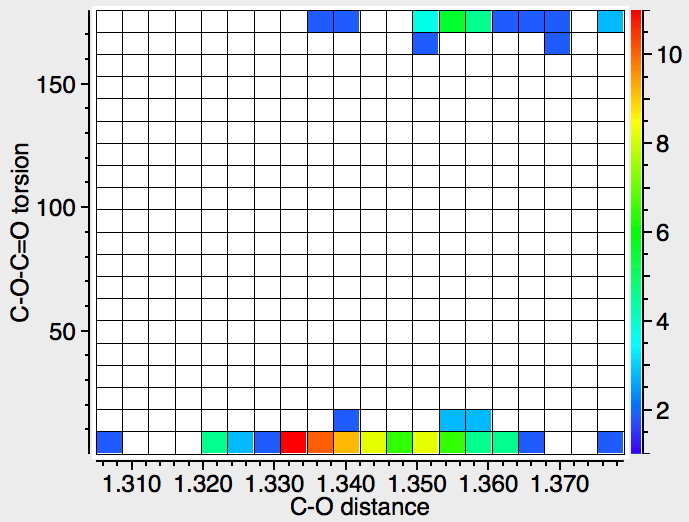
Repeating the search, but specifying that the temperature of data acquisition is < 90K, one gets a much clearer indication of the difference in bond lengths.
This alternative representation shows the C-O and the C=O distances, with red indicating s-trans and blue indicating s-cis conformations (T < 140K). The red dots occupy a bottom right cluster for which the C-O distance is longer and the C=O shorter than the corresponding blue cluster.
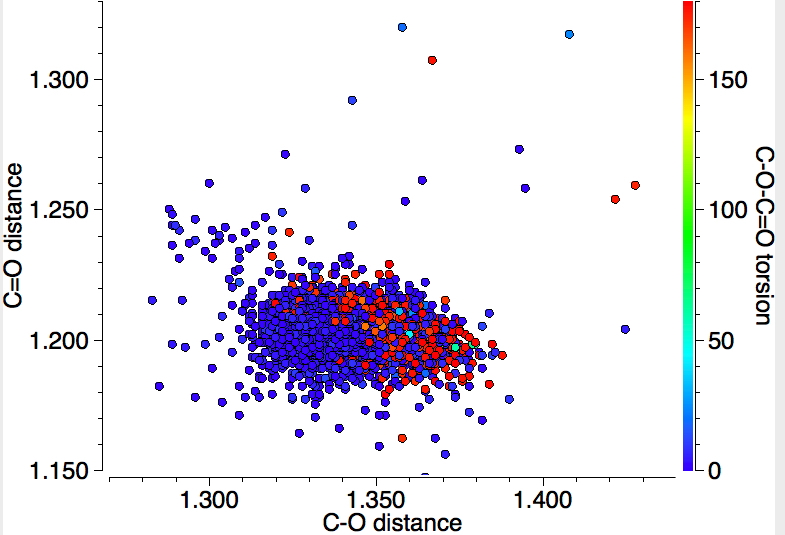
Again reducing the temperature of data collection to < 90K shows a rather weak inverse correlation between the two distances for eg the blue dots.
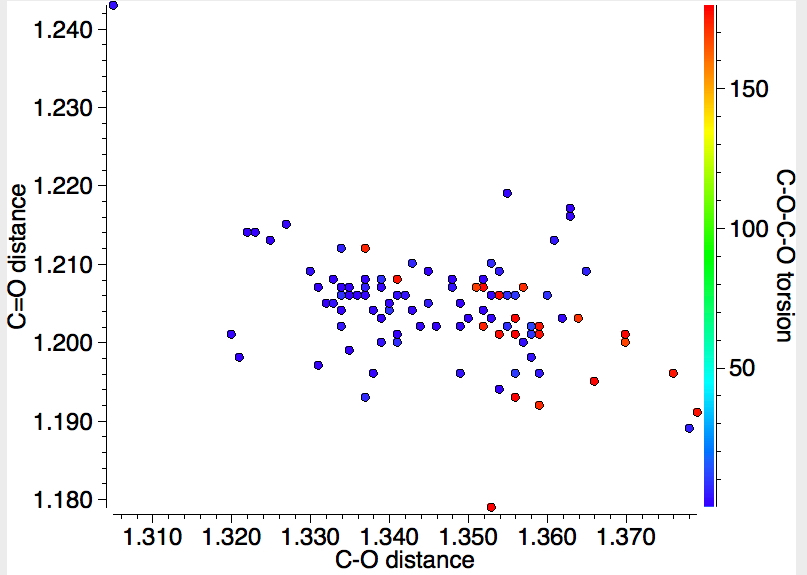
A shame however that this database does not hold IR values for the carbonyl stretches. I am sure correlations must exist, but how to get at them (other than manual collection of data).
Tags: Ester, Functional groups, Infra-Red
Here is a plot in which the C=O of the ester is replaced by C=CRR’ (where R or R’ can be either H or a 4-coordinate carbon). In addition the C-O-C= is replaced by R-O-C=, where R can be either 4-coordinate carbon or silicon. This is now an enol rather than an ester.
The effect is less clear-cut for these enols, largely because there are only 71 examples of crystal structures for such systems. Nevertheless, the red dots (s-trans) are mostly clustered in the bottom right and the blue dots (s-syn) mostly in the top left, just as with the esters.