In the first part of the post on this topic, I described how an asymmetric sulfoxide could be prepared as a pure enantiomer using a chiral oxygen transfer reagent. In the second part, we now need to deliver a different group, cyano, to a specific face of the previously prepared sulfoxide-imine. The sulfoxide is now acting as a chiral auxilliary, and helps direct the delivery of the cyanide group to specifically one face of the imine rather than the other. After removal of the aluminum carrier for the cyano group and hydrolysis of the cyano group to a carboxylic acid group, we end up with an enantiomerically pure amino acid.

The Strecker synthsis: asymmetric delivery of cyanide anion. Click for 3D model of transition state

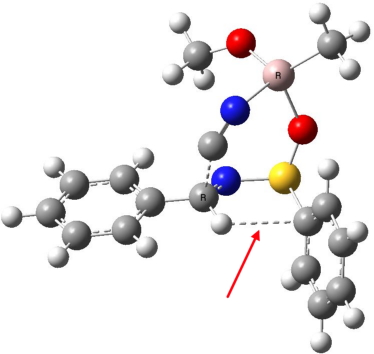
AIM analysis for the asymmetric delivery of cyanide to an imine, S,S(S) form.
The take home message from these two posts is that quite unusual interactions may often be responsible for asymmetric induction in a stereospecific reaction, and that helpful clues to these interactions may well be derived from an AIM analysis. Indeed, anyone doing stereospecific synthesis in the lab should be familiar with these methods! You have to be a jack-of-all-trades nowadays to keep up!
Tags: aluminum carrier, carboxylic acid, chiroptical, cyano, free energy, Interesting chemistry