If, as a synthetic chemist, you want to invert the configuration of an alcohol in which the OH group is at a chiral centre, then the Mitsunobu reaction has been a stalwart for many years. Now a catalytic version has been published, [1] along with a proposed mechanism. Here I apply computation as a reality check to see what the energetics of this mechanism might be.
The chosen computational procedure was B3LYP+GD3BJ dispersion, Def2-TZVPP basis and SCRF=toluene as solvent and for the alcohol, R1=R2=Me. Data is at DOI: 10.14469/hpc/6186
| Molecule | ΔΔG, kcal/mol |
|---|---|
| 1 | 0.0 |
| 2 | 25.4 |
| 3 | 15.0 |
| TS | 36.8 |
| 4 | +4.5 |
In the mechanism above, the proton transfers have not been included in the modelling, with the presumption that the step involving the inversion at carbon is rate limiting.
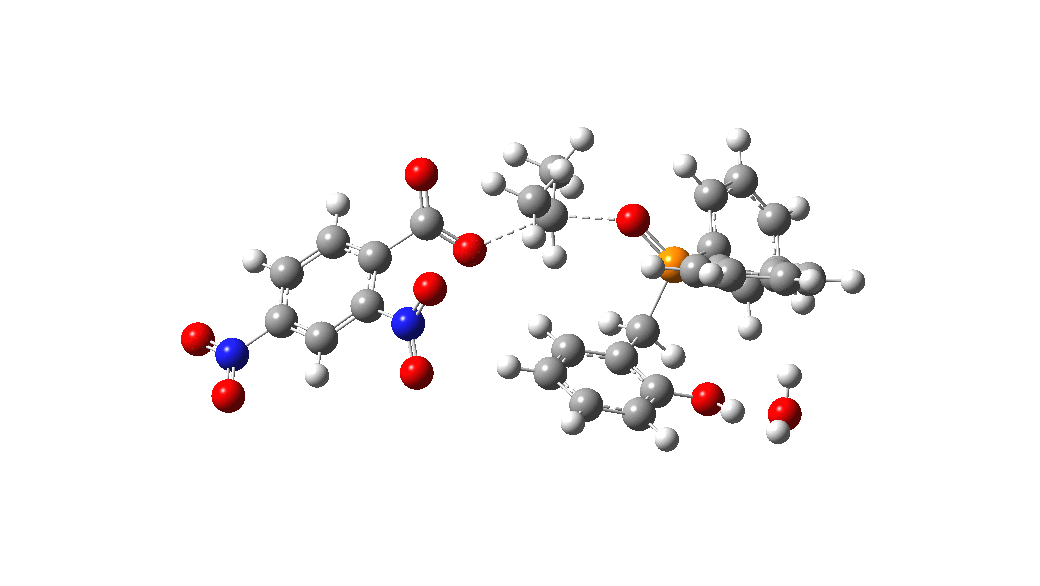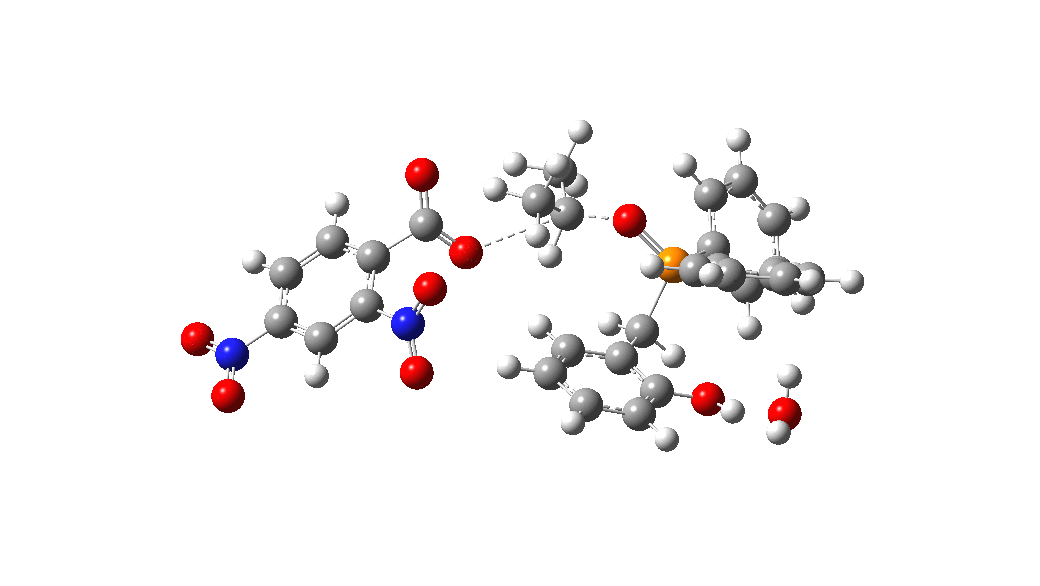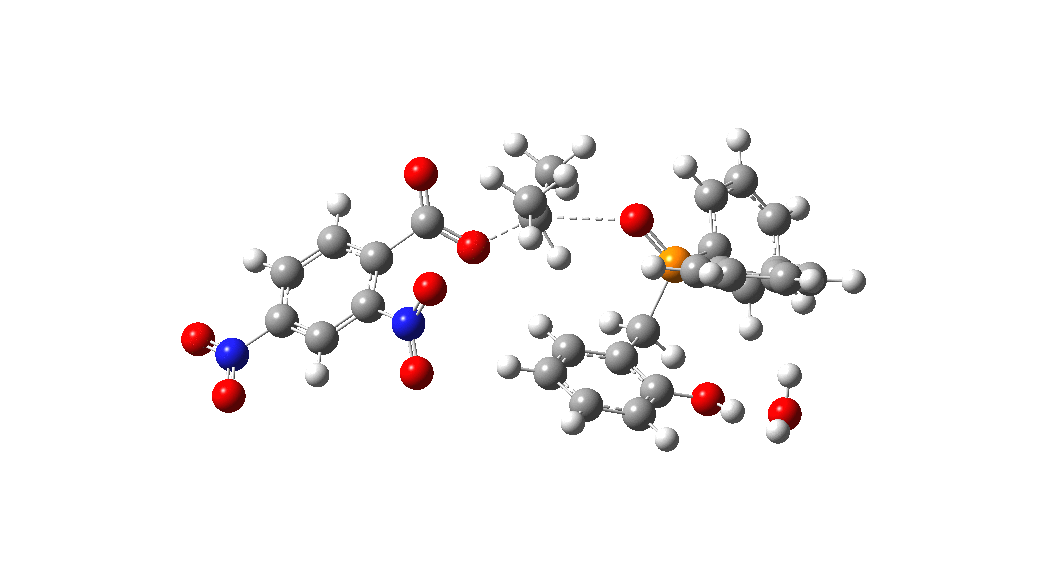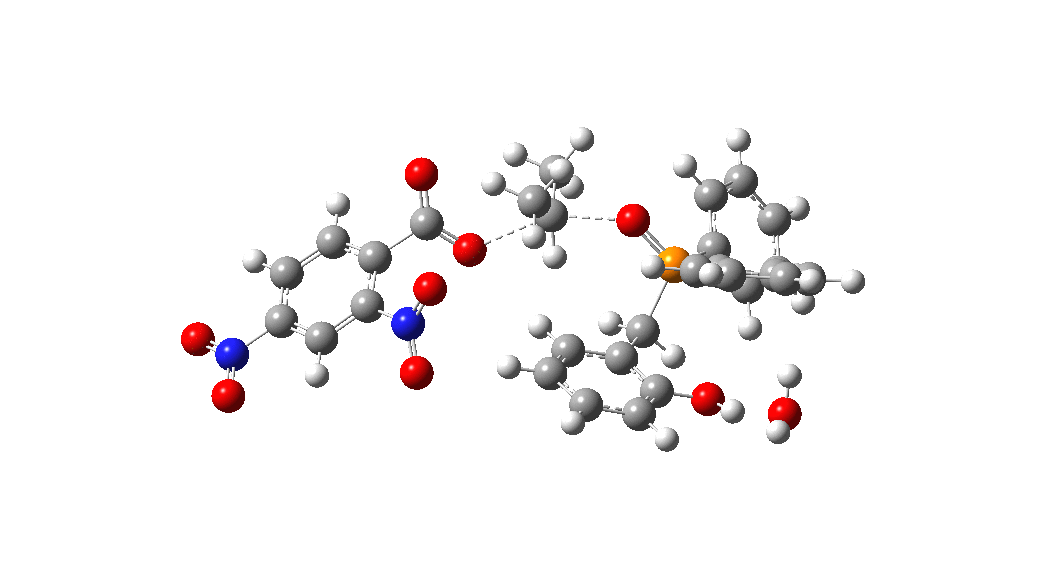 Overall, the reaction is slightly endoenergic, and this is supported by the need to azeotropically remove water from the reaction. The activation barrier for TS from species 1 however is perhaps somewhat high for a reaction occuring in refluxing toluene. Annoyingly so, since high barriers are sometimes indicative that the mechanism modelled is missing some key aspect. So there must be (a small?) possibility that a more complex mechanism might operate in this catalytic cycle, or that a systemic error in the DFT (density functional theory) approach might be present.
Overall, the reaction is slightly endoenergic, and this is supported by the need to azeotropically remove water from the reaction. The activation barrier for TS from species 1 however is perhaps somewhat high for a reaction occuring in refluxing toluene. Annoyingly so, since high barriers are sometimes indicative that the mechanism modelled is missing some key aspect. So there must be (a small?) possibility that a more complex mechanism might operate in this catalytic cycle, or that a systemic error in the DFT (density functional theory) approach might be present.
References
- R.H. Beddoe, K.G. Andrews, V. Magné, J.D. Cuthbertson, J. Saska, A.L. Shannon-Little, S.E. Shanahan, H.F. Sneddon, and R.M. Denton, "Redox-neutral organocatalytic Mitsunobu reactions", Science, vol. 365, pp. 910-914, 2019. https://doi.org/10.1126/science.aax3353