Having shown that carbon as a carbene centre, C: can act as a hydrogen bond acceptor, as seen from a search of crystal structures, I began to wonder if there is any chance that carbon as a radical centre, C• could do so as well. Definitely a subversive thought, since radical centres are supposed to abstract hydrogens rather than to hydrogen bond to them.
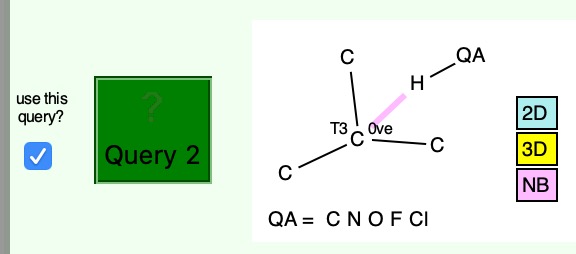
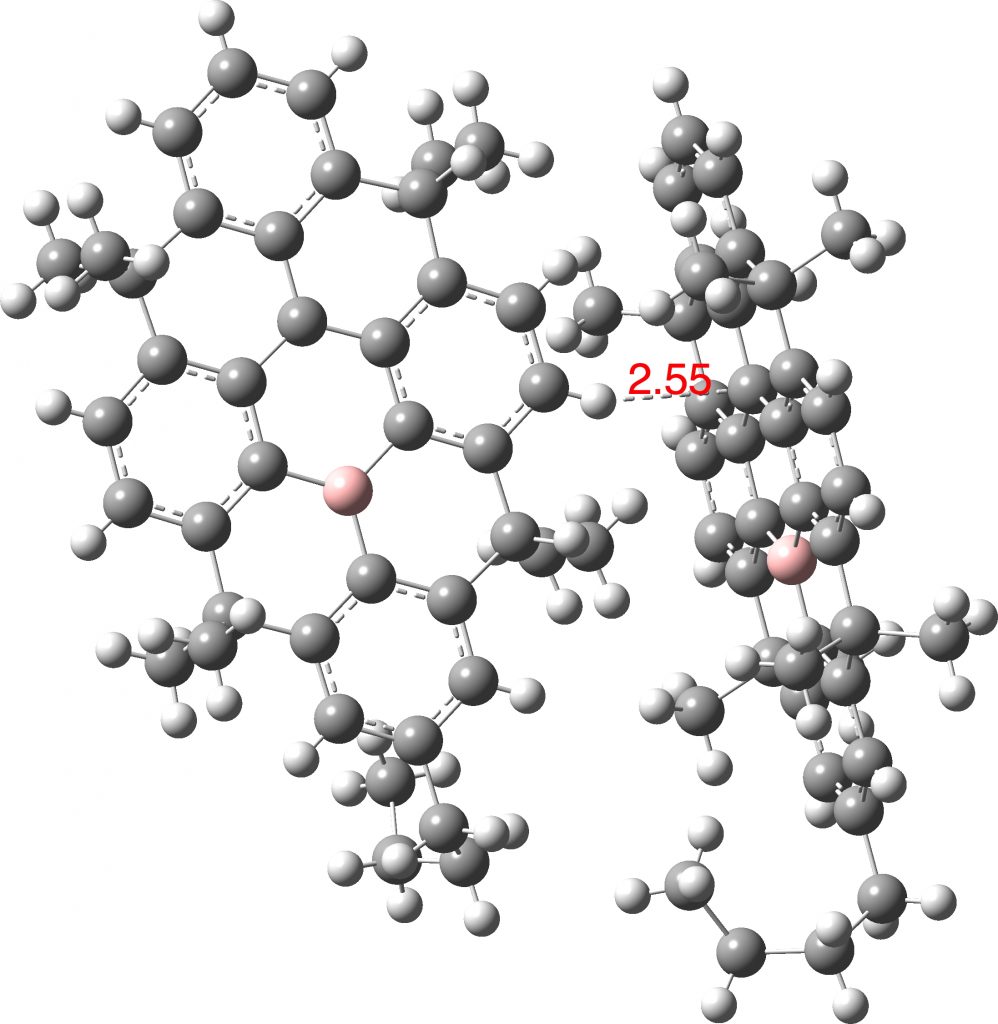
 One molecule that emerges from such a search (Query: 10.14469/hpc/6572) was reported recently as a resonance stabilized radical,[1] with the intermolecular hydrogen bond that emerges being from an aryl C-H directed at the carbon radical centre. The length (after correction by -0.1Å) is typical (this interaction is not noted in the article itself). Most of the 31 hits are in fact intra-molecular.
One molecule that emerges from such a search (Query: 10.14469/hpc/6572) was reported recently as a resonance stabilized radical,[1] with the intermolecular hydrogen bond that emerges being from an aryl C-H directed at the carbon radical centre. The length (after correction by -0.1Å) is typical (this interaction is not noted in the article itself). Most of the 31 hits are in fact intra-molecular.
Click image to view 3D model
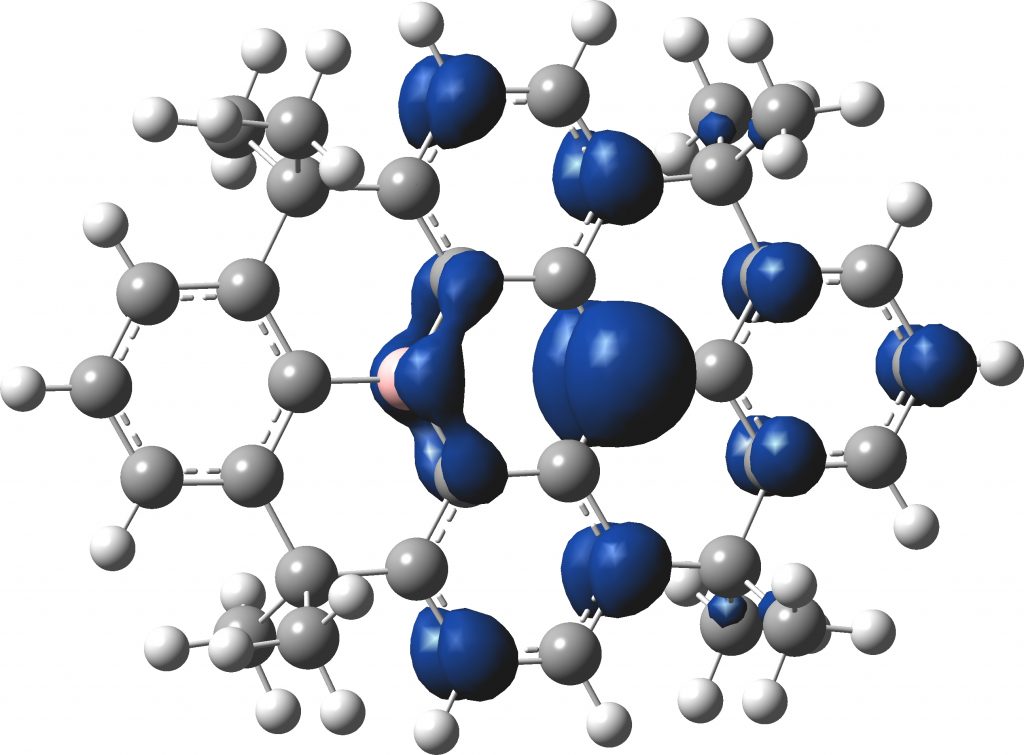
The spin density arising from the unpaired electron of the radical is indeed delocalised, although the largest part is in a pπ orbital on the carbon radical centre.
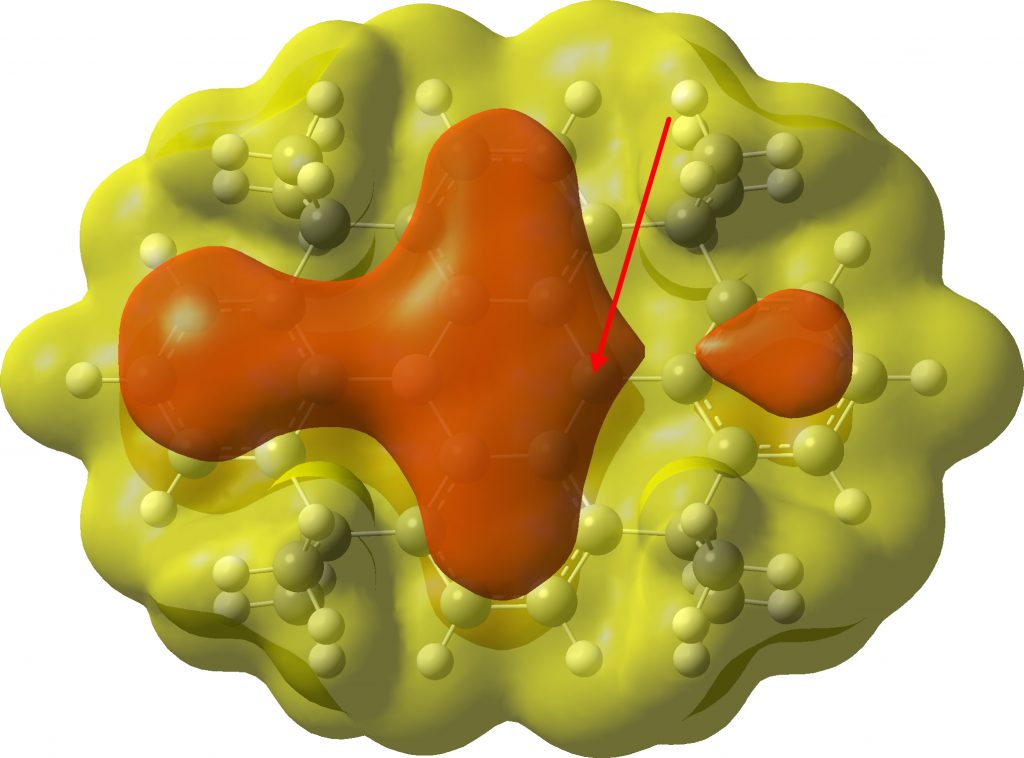
The molecular electrostatic potential (MEP) shows a negative π-potential (attractive to a proton) covering the radical carbon, but also the adjacent boron.
These types of hydrogen bond to a carbon radical acceptor are clearly weak (if indeed they are real), but perhaps a balance has to be achieved between two effects: less delocalised carbon radicals might form stronger hydrogen bonds but they will also abstract hydrogen atoms from potential hydrogen bond donors. More highly delocalised radicals are less likely to abstract, but probably also less likely to form strong hydrogen bond acceptors. Nonetheless, one can ask whether a stronger carbon radical hydrogen bond acceptor might be found that exists in that region where abstraction does not occur. As I noted at the start, I am trying to be provocative!
References
- T. Kushida, S. Shirai, N. Ando, T. Okamoto, H. Ishii, H. Matsui, M. Yamagishi, T. Uemura, J. Tsurumi, S. Watanabe, J. Takeya, and S. Yamaguchi, "Boron-Stabilized Planar Neutral π-Radicals with Well-Balanced Ambipolar Charge-Transport Properties", Journal of the American Chemical Society, vol. 139, pp. 14336-14339, 2017. https://doi.org/10.1021/jacs.7b05471