The title of this post merges those of the two previous ones. The tunable C-Cl bond brought about in the molecule tris(amino)chloromethane by anomeric effects will be probed using the Laplacian of the electronic density.

Laplacian @0.67 for tris(amino)choromethane. Click for 3D
The figure above shows the Laplacian for a conformation of tris(amino)chloromethane with one of the nitrogen lone pairs antiperiplanar to the C-Cl bond, and the other two lone pairs antiperiplanar to C-N bonds. The features visible at an isosurface of ± 0.67 include
- (a) The Laplacian here has a value of -0.67 (= red isosurface), which indicates an accumulation of (covalent) shared density along the C-N bond (underneath this surface, you can see the blue sphere representing depletions from the nitrogen atomic region). This bond has the lone pair antiperiplanar to a C-N bond.
- (b) Contrast this with the C-N bond which is antiperiplanar to the C-Cl bond. A greater volume of the covalent C-N region is bounded by this isosurface. More of the N lone pair on this atom is donating into the C-N, as more conventionally represented below.
- Notice how the red isosurface associated with the N lone pair and the region associated with the C-N bond are in fact contiguous, and not separated basins!
- (c) represents the lone pairs on the chlorine, which have been augmented by the donation from the nitrogen. Notice how they come out as a torus rather than the conventional double dot representations!
- Notice the absence of any features along the C-Cl bond! This would be typical of a fully or even partially ionic bond, but it also illustrates that with a property such as the Laplacian, one does not get a complete picture by inspecting at just one isosurface value.
The next isosurface chosen is 0.3. At this lower value, more depletions (blue = electrophilic regions) are seen and a tiny feature now appears along the C-Cl bond, which is the covalent accumulation of that bond, a feature that grows @ 0.2. This nicely illustrates the variable covalency/ionicity of the C-Cl bond. Notice also how the lhs is all red (anionic) and the rhs is mostly blue (cationic), showing the formation of in effect an ion pair.
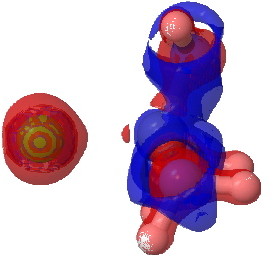 tris(amino)chloroethane @ 0.3 |
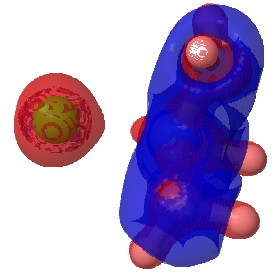 tris(amino)chloroethane @ 0.2 |
There are many other features which can be explored in these Laplacian maps, but I leave those for the reader to indulge in. Just click on any of the diagrams above,and start your exploration.
Tags: Anomeric effect, Chemical Energy, Interesting chemistry, Laplacian