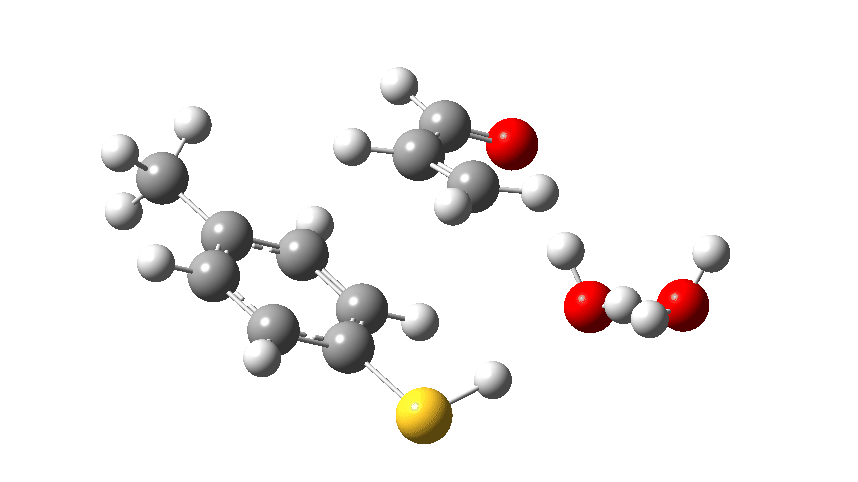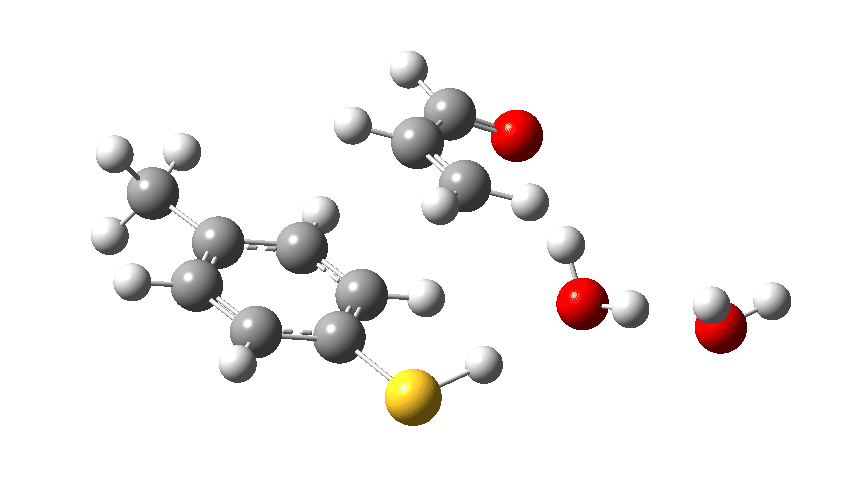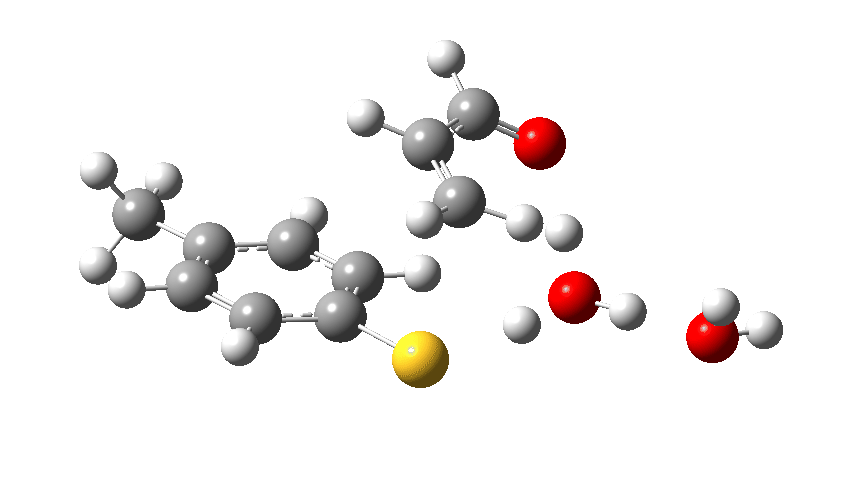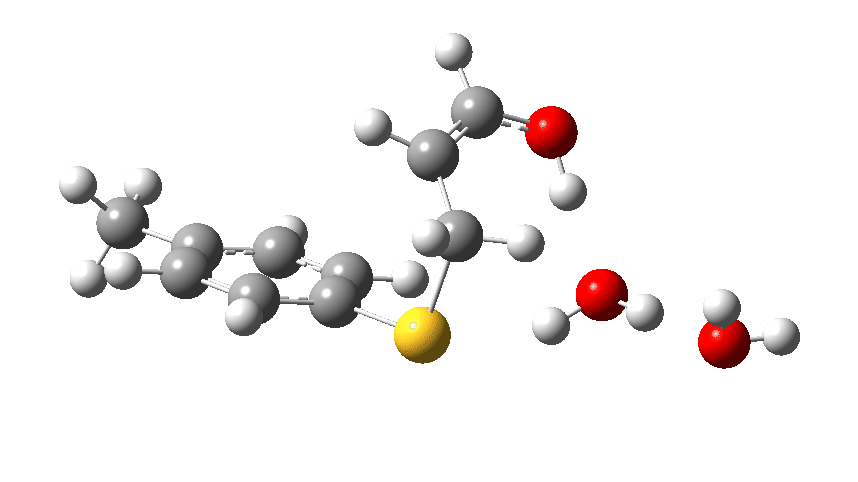
A reaction can be thought of as molecular dancers performing moves. A choreographer is needed to organise the performance into the ballet that is a reaction mechanism. Here I explore another facet of the Michael addition of a nucleophile to a conjugated carbonyl compound. The performers this time are p-toluene thiol playing the role of nucleophile, adding to but-2-enal (green) acting as the electrophile and with either water or ammonia serving the role of a catalytic base to help things along.†
The scheme above is deliberately set out as an eight-membered ring so that if the three dancers wish to do so, they can all act in concert. Oh, there is also a bit-actor (water) forming a hydrogen bond to X, the role of which will become clearer as the ballet proceeds. The curly arrows indicate what the electrons in the bonds or the lone pairs are expected to do. The three black arrows can be accompanied by either two blue arrows to give five in all, or just four if the two blue arrows are replaced by a single red one.
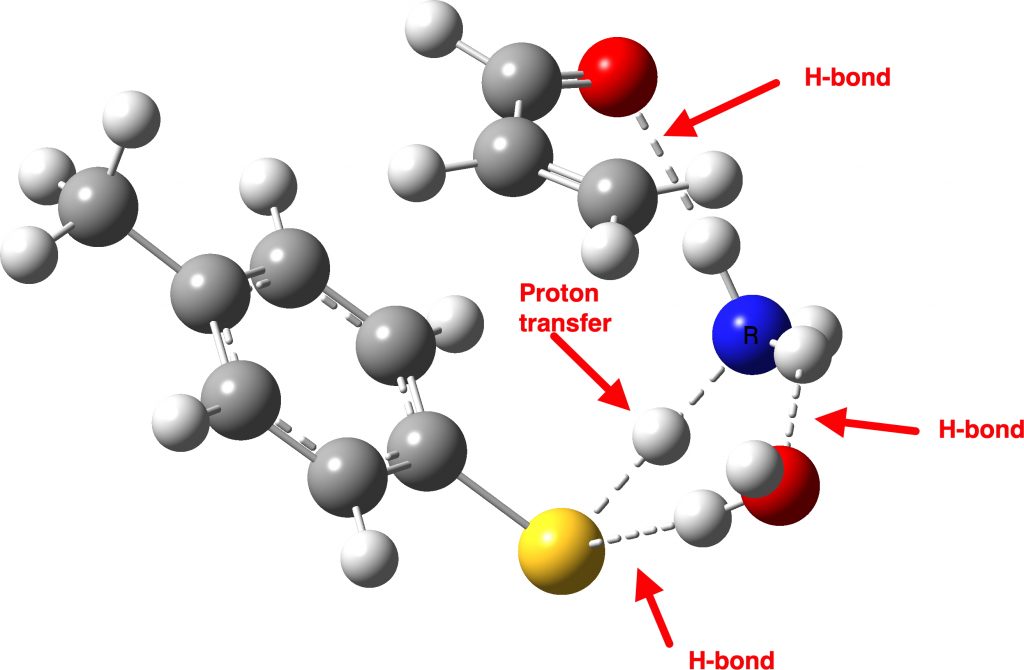
The choreographer in our performance is actually going to be a density functional quantum mechanical calculation (ωB97XD/Def2-TZVPP/SCRF=water, data at DOI: 10.14469/hpc/7027 since you ask), which has the single minded intention of ensuring that the cast is at the lowest possible energy at each stage of the ballet. The performance is shown below with X=O in the cast (water). Water is a poor base; its ability to grab a proton is weak.
We can also show the entire dance using an Intrinsic Reaction Coordinate or IRC, this being the lowest energy pathway that the cast can achieve along this particular route to the end. Watch the animation above to see the performance! The catalyst (X=O remember) firstly gets into the best position to grab a proton from the S-H group, using its lone pair located on the oxygen (the base). It is helped by the bit-playing second water molecule, which forms an assisting support to the (lets call her) ballerina via a strong hydrogen bond. Having grabbed the proton from the ballerino, the catalyst transforms (temporarily) into a hydronium cation, paired now with a thiolate anion as an ion-pair. Temporarily, because this sort of arrangement is called a “hidden intermediate” in that this ion-pair is hidden, never actually forming. The water needs considerable help to become protonated (remember, it is a weak base), with the assisting water bit-player helping to stabilize the hydronium cation by a strong hydrogen bond it has formed.
The transition state for the reaction. Click to view 3D model. The vibration is that of the “transition state normal mode” as the molecule goes over the top of the barrier.
We now introduce the (relative) energy of the entire collection of molecules and have reached the stage of IRC=-1 on the X-axis. One final push is now needed, in which two things happen. Firstly, a S-C bond is formed (IRC = 0.0, the transition state) but as soon as it starts forming so does the rather unhappy hydronium cation relieve itself of the unwelcome proton it just acquired, by off-loading it onto the oxygen of the acrolein. You can see the structure of this transition state above (click on the image to turn it into a rotatable 3D model)
The catalyst is back to where it started (along with its bit-playing partner) and we now have a completed reaction and it all happened as a single act ballet (we call this a concerted performance). The products are lower in energy than the starting point, which is always good! Molecules tend to be lazy and do not much like becoming higher in energy (ATP, or adenosine triphosphate is a famously unlazy molecule which is very good at acquiring lots of energy and redistributing it about our bodies to feed our muscles).
We can look at another property which tells us a bit more about the curly arrows, which represent rearrangement of electrons within the molecule. If they get separated, their charges also become separated and this is reflected in the dipole moment along the reaction coordinate. In the early stages, blue arrow 1 starts to form a hydrogen bond from the lone pair of the water to the hydrogen on the S. As it does this, the dipole moment decreases. At the point that the proton finally decided to hop from the sulfur to the approaching water oxygen, the charge separation shoots up, reaching its maximum at IRC = -1 (IRC = 0 by the way represents the energy high point for the process, called the transition state).
I want now to address the vital point of why I drew two different arrangements of curly arrows, one with two blue arrows (1 and 5) and the other with just one red arrow (6). If we had instead used just the latter, then we would have been obliged to transfer both protons at exactly the same time. So blue arrow 1 is a better representation of what is actually going on. Only now do the black arrows 2–4 get into the performance, forming the S-C bond (2), reducing the first double bond in the acrolein to single, whilst reforming it adjacently (3) and transforming the second C=O double bond into C-O and O-H bonds (4). This encourages the second blue arrow (5) to, concurrently with the black arrows, transfer a proton and reform the lone pair onto the original oxygen of the water catalyst.
Let us now change the cast, replacing the original water catalyst with an ammonia (X=NH). Because N has a smaller nuclear charge than oxygen, it is happier at sharing its lone pair with a proton; it is said to be more basic. This means that an ammonium cation is a more willing performer than the hydronium cation. The ballet now occurs in two acts rather than one. The first act involves that now basic nitrogen removing the proton from the SH (arrow 1+2), but with arrow 2 ending up residing entirely on the S (as a sulfur lone pair) rather than immediately going on to form a S-C bond.
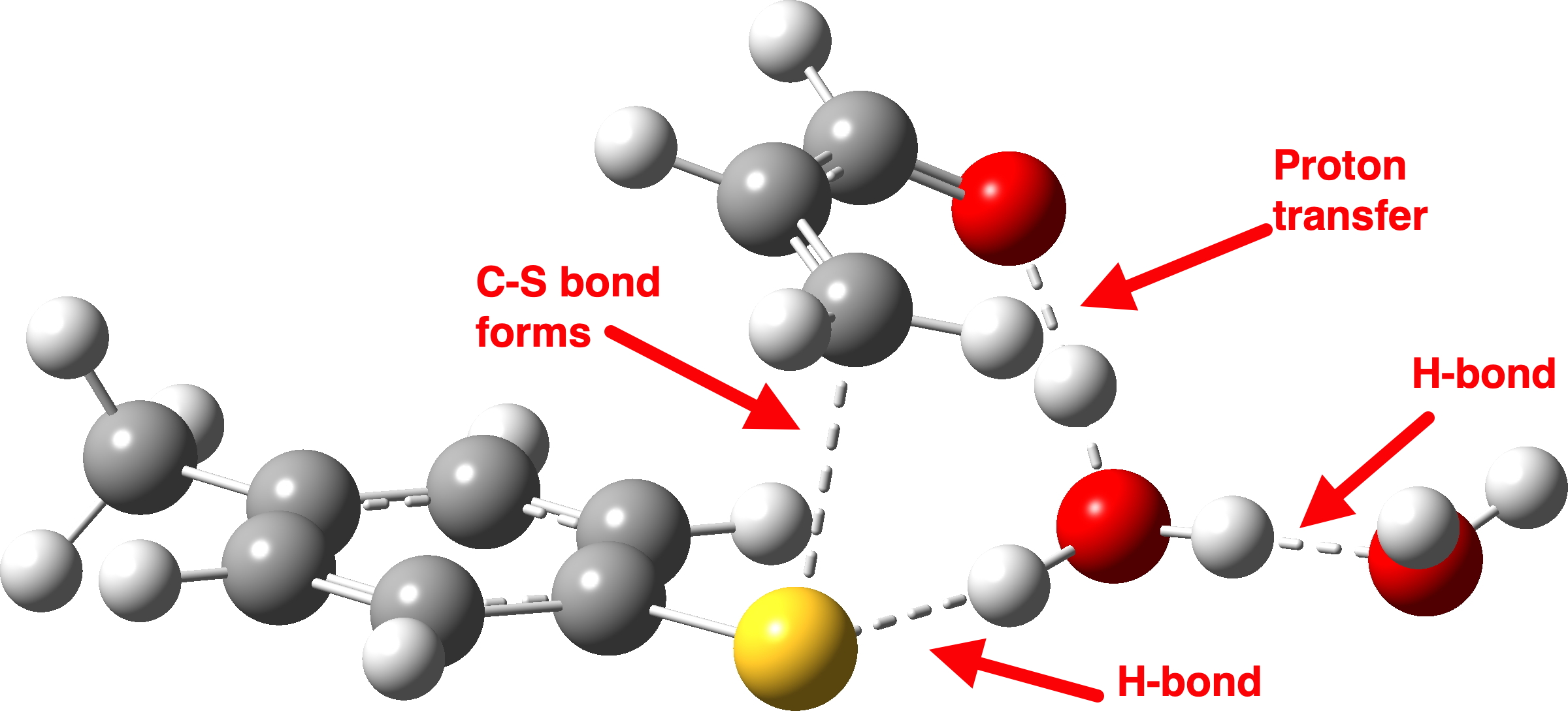
There is then an intermission when the newly formed ion-pair takes a break, followed by the second act starting with a slightly different arrow 2 (it starts not at the S-H bond, but put on a new costume during the break to start as a new lone pair formed on the S) creating the new S-C bond. There is another difference compared to the water catalyst; the ammonium cation is now slightly reluctant to relinquish that proton and this only happens right at the end.
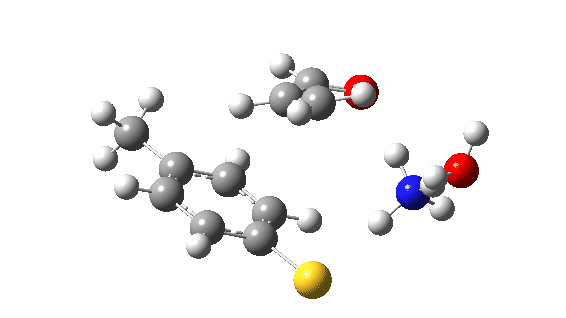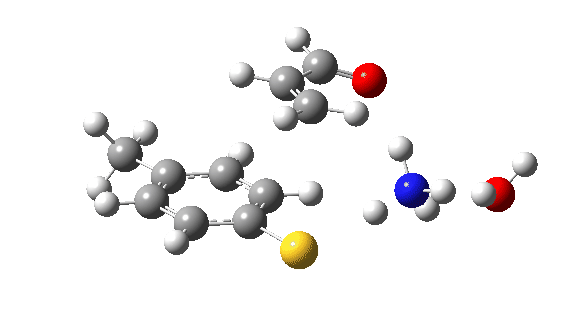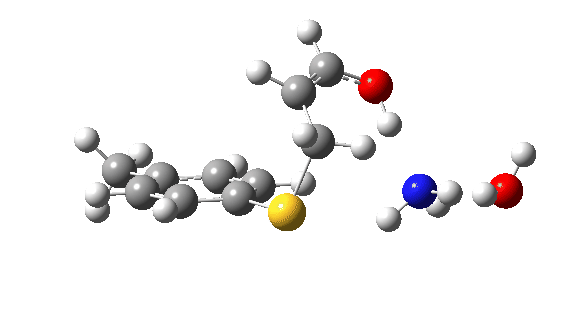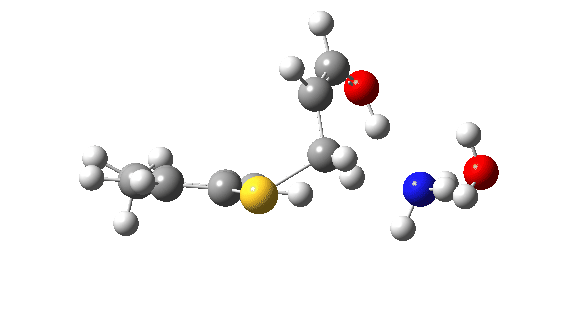
Act 2: Carbon-sulfur bond formation/Proton transfer. Click to view 3D model.
The energy high point is again S-C bond formation (IRC = 0.0), and the barrier the molecules needed to overcome to reach the energy high point is much lower than before. The nitrogen hangs on to its newly acquired proton until IRC = -2 and the reaction does look complete by IRC = -10. But in a final flourish (let’s call it an encore‡) something happens between IRC -10 to -15. Miffed at having to part with a hydrogen it had become fond of, the nitrogen lone pair instead now makes friends with a C-H bond (as part of a hydrogen bond; it is not basic enough to entirely remove a hydrogen from a carbon).
The language has been slightly anthropomorphic, but we have covered a lot of chemistry with this reaction and learnt a lot about the sequence in which bonds form and how curly arrows can be used to relate to this process.
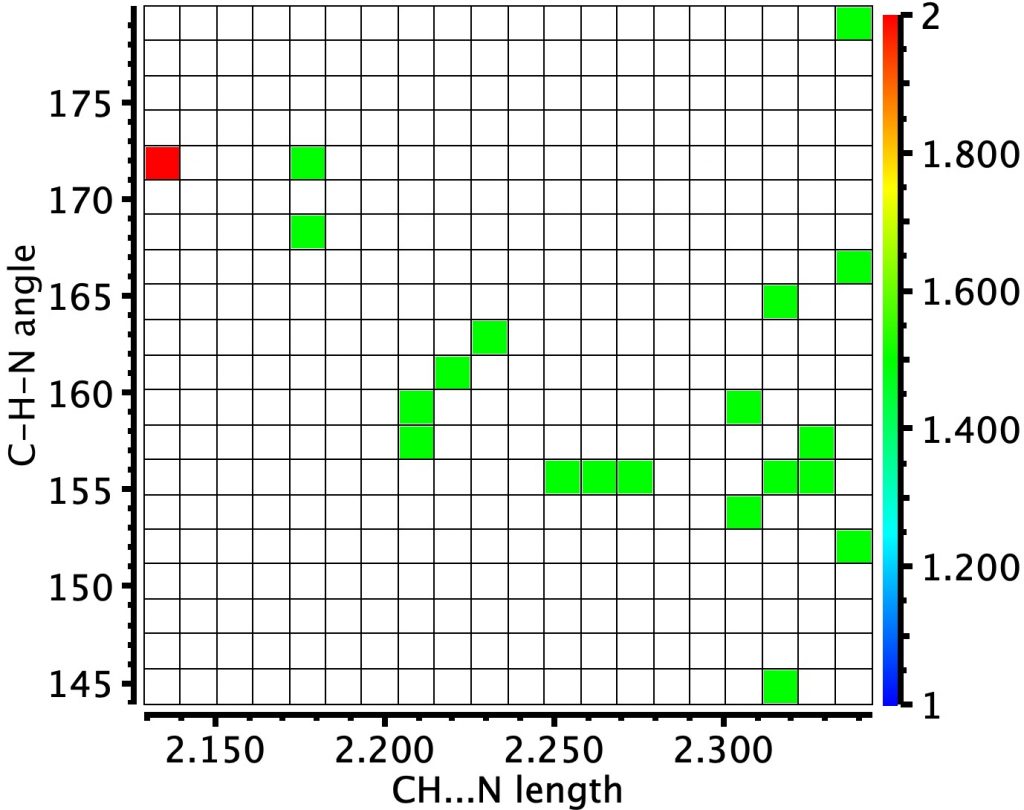
‡The encore: We can check to see if this last part comes purely from the fevered imagination of the density functional calculation or whether there is a basis in reality for this new friendship. The plot below comes from a search of all known crystal structures for organic molecules (which recently passed one million). Of these, 21 exhibit a CH…N distance < 2.45Å and the “hotspot” (in red) indicates that the strongest of these is ~2.15Å and that the C-H…N angle is approximately linear. So the effect is real!
†See also this post for the non-catalysed version of this reaction.
This post has DOI: http://doi.org/dr96