Zosurabalbin[1],[2], is receiving a great deal of attention as a new class of antibiotic which can target infections for which current treatment options are inadequate. It is a cyclic peptide and seeing this triggered memory of an earlier such species reported way back in 1995[3],[4]. This octa-peptide (YIJDIE, DOI: 10.5517/cc58gxs) was presumed to function in a novel manner, having linear water channels wide enough to form a molecular nanoscale pipe for a stream of water molecules to flow along. When inserted into the bacterial cell membrane via its lipophilic sidechains, it drained the bacterium of its cell water within seconds, thus killing it. A 3D model shows the effect very clearly.
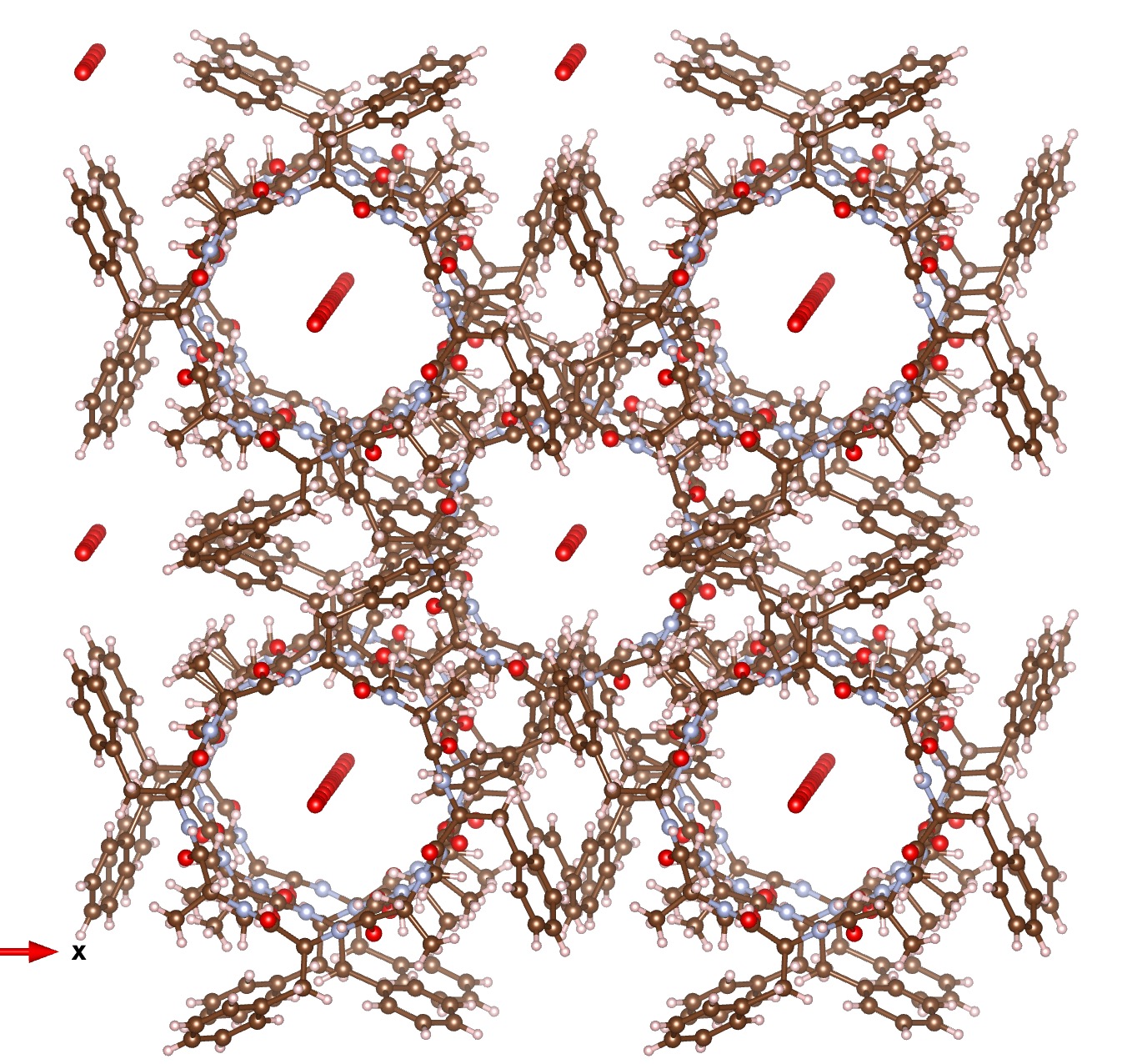
Zosurabalpin does not function in this manner. Its structure was devised by optimising the various substituents until optimal activity was obtained (see this patent WO202319441).
The ligand (VB6) is seen below. A program such as Chimera can tease out many more details.
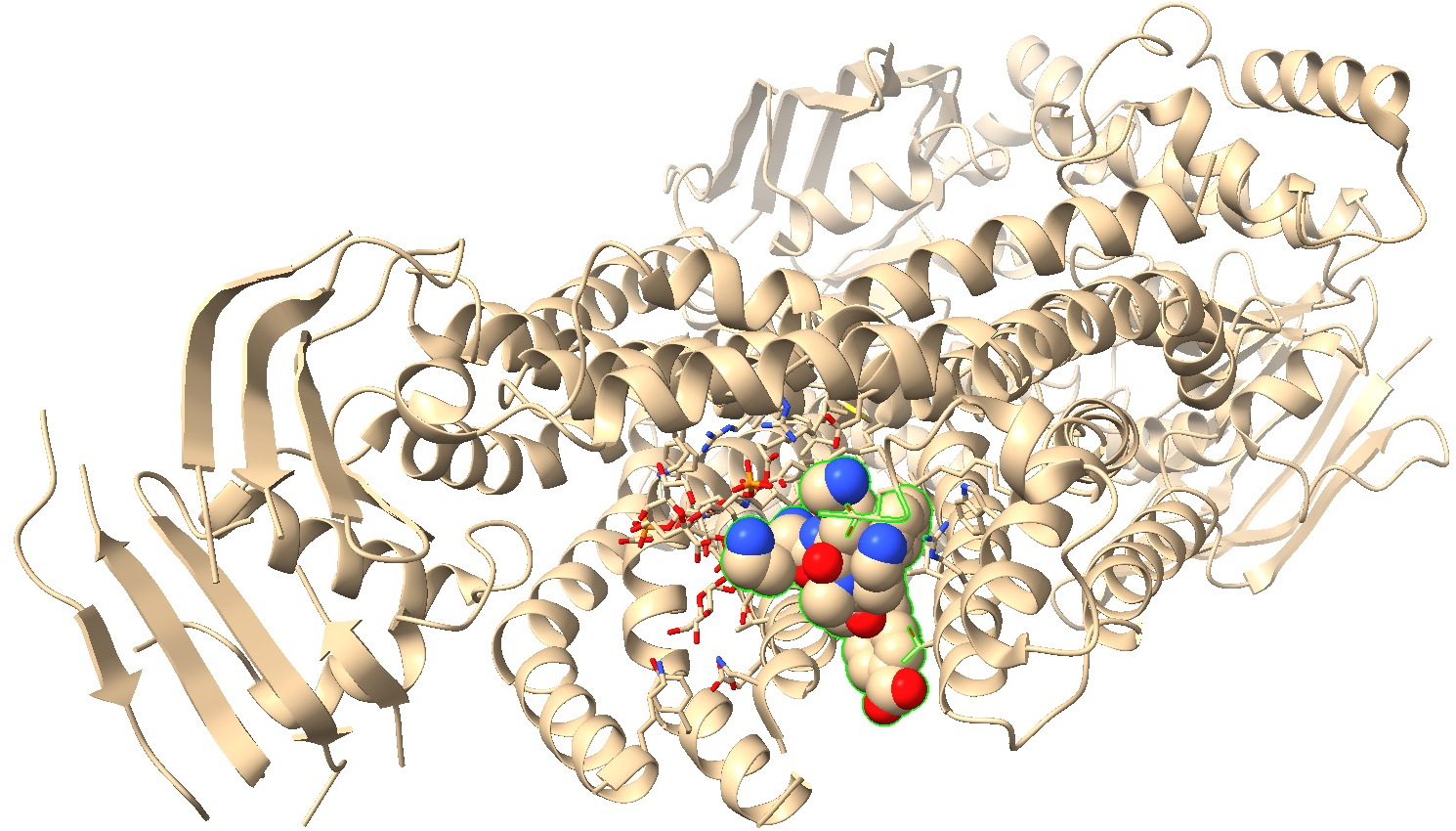
Zosurabalpin embedded in the protein pdb8frn can be viewed below and the coordinates can be obtained via DOI: 10.2210/pdb8frn/pdb

The original 1995 report[3] about the cyclic octapeptide appears was never developed into a clinically useful antibiotic, but I wonder where this approach led to.
References
- C. Zampaloni, P. Mattei, K. Bleicher, L. Winther, C. Thäte, C. Bucher, J. Adam, A. Alanine, K.E. Amrein, V. Baidin, C. Bieniossek, C. Bissantz, F. Boess, C. Cantrill, T. Clairfeuille, F. Dey, P. Di Giorgio, P. du Castel, D. Dylus, P. Dzygiel, A. Felici, F. García-Alcalde, A. Haldimann, M. Leipner, S. Leyn, S. Louvel, P. Misson, A. Osterman, K. Pahil, S. Rigo, A. Schäublin, S. Scharf, P. Schmitz, T. Stoll, A. Trauner, S. Zoffmann, D. Kahne, J.A.T. Young, M.A. Lobritz, and K.A. Bradley, "A novel antibiotic class targeting the lipopolysaccharide transporter", Nature, vol. 625, pp. 566-571, 2024. https://doi.org/10.1038/s41586-023-06873-0
- S. Hawser, N. Kothari, T. Valmont, S. Louvel, and C. Zampaloni, "2131. Activity of the Novel Antibiotic Zosurabalpin (RG6006) against Clinical <i>Acinetobacter</i> Isolates from China", Open Forum Infectious Diseases, vol. 10, 2023. https://doi.org/10.1093/ofid/ofad500.1754
- M.R. Ghadiri, K. Kobayashi, J.R. Granja, R.K. Chadha, and D.E. McRee, "The Structural and Thermodynamic Basis for the Formation of Self‐Assembled Peptide Nanotubes", Angewandte Chemie International Edition in English, vol. 34, pp. 93-95, 1995. https://doi.org/10.1002/anie.199500931
- S. Fernandez-Lopez, H. Kim, E.C. Choi, M. Delgado, J.R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D.A. Weinberger, K.M. Wilcoxen, and M.R. Ghadiri, "Antibacterial agents based on the cyclic d,l-α-peptide architecture", Nature, vol. 412, pp. 452-455, 2001. https://doi.org/10.1038/35086601