Michael in a comment here on the mechanism of the Masamune-Bergman reaction notes that when it occurs as part of the Calicheamicin (an antibody-drug conjugate or ADC) version of this mechanism, a pre-step is first necessary. As discussed in this review article,[1] the trisulfide linkage is reduced and the resulting thiolate undergoes a facile 1,4-addition to the adjacent enone.
DFT calculations on the new form (FAIR Data DOI:[2], [2] show that the free energy barrier is reduced from 38.6 kcal/mol to 26.2 kcal/mol.
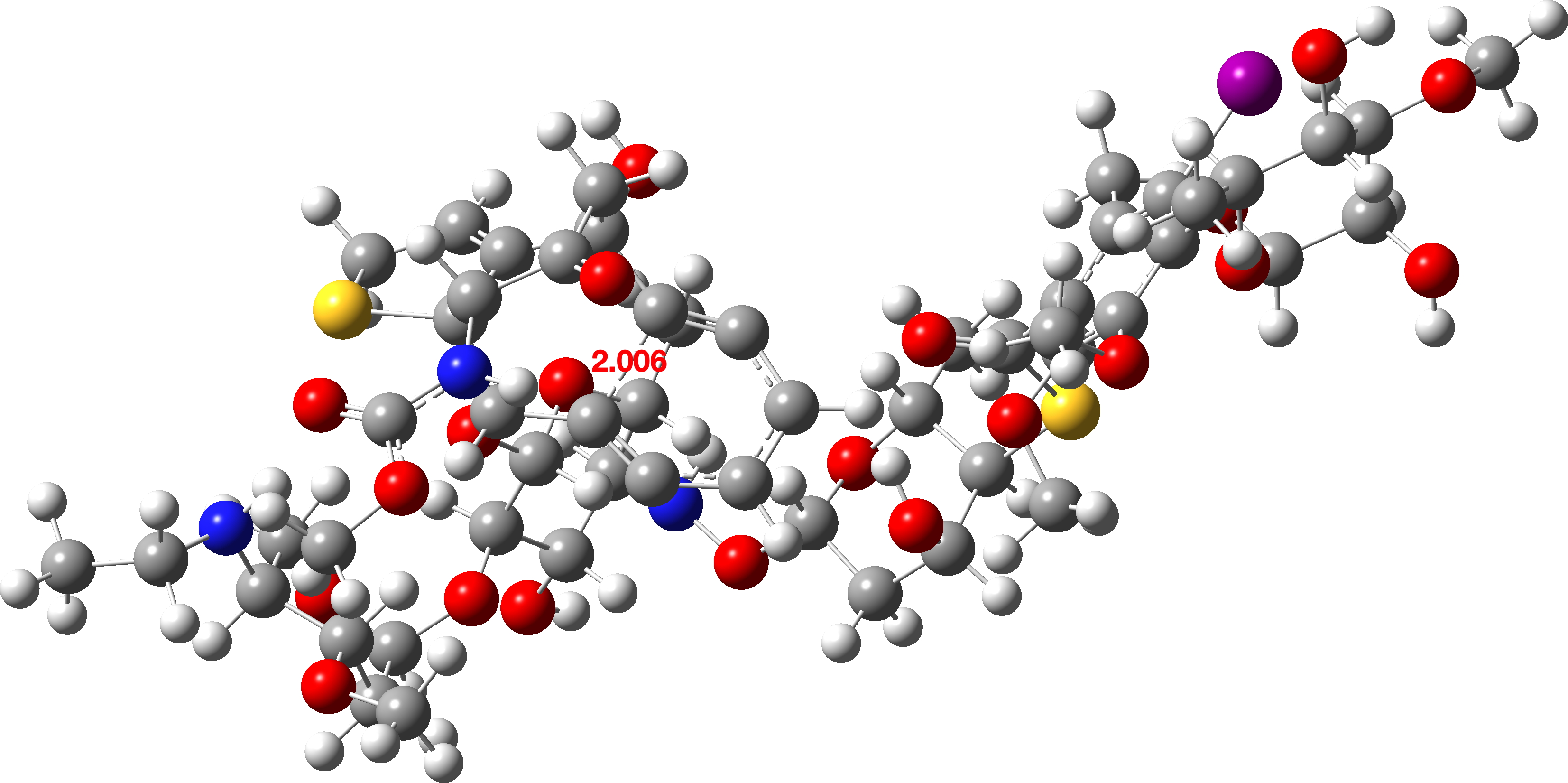
This is now a reasonable value for a thermal reaction, being a 12.4 kcal/mol reduction from the unactivated species. We can conclude that Michael’s suggestion was spot on, and suggests in turn that a DFT-biradicaloid calculation is in fact a reasonable procedure for modelling this type of system.
References
- V. Kostova, P. Désos, J. Starck, and A. Kotschy, "The Chemistry Behind ADCs", Pharmaceuticals, vol. 14, pp. 442, 2021. https://doi.org/10.3390/ph14050442
- H. Rzepa, "Mechanism of the Masamune-Bergman reaction. Part 4. Why is the DFT energy barrier too high?", 2024. https://doi.org/10.14469/hpc/14632