Derek Lowe tells the story of “carbyne”, a potential further allotrope of carbon, comprising linear chains of carbon atoms, C-C≡C-C≡C-C. Whether such a molecule can exist on its own has long been the the topic of speculation. Now a report has appeared of a “pseudocarbyne”, stabilised by gold atoms.[1]
The now thankfully almost ubiquitous data availability statement includes the DOI: https://doi.org/10.48349/ASU/3TWEI0 [2] as a data repository source of replication data and one of the files found there is a CIF containing the crystal data. Playing with this, I noticed one unusual feature of this structure, which oddly is not apparently mentioned in the article itself and so I thought I would tease it out here – 12 coordination.
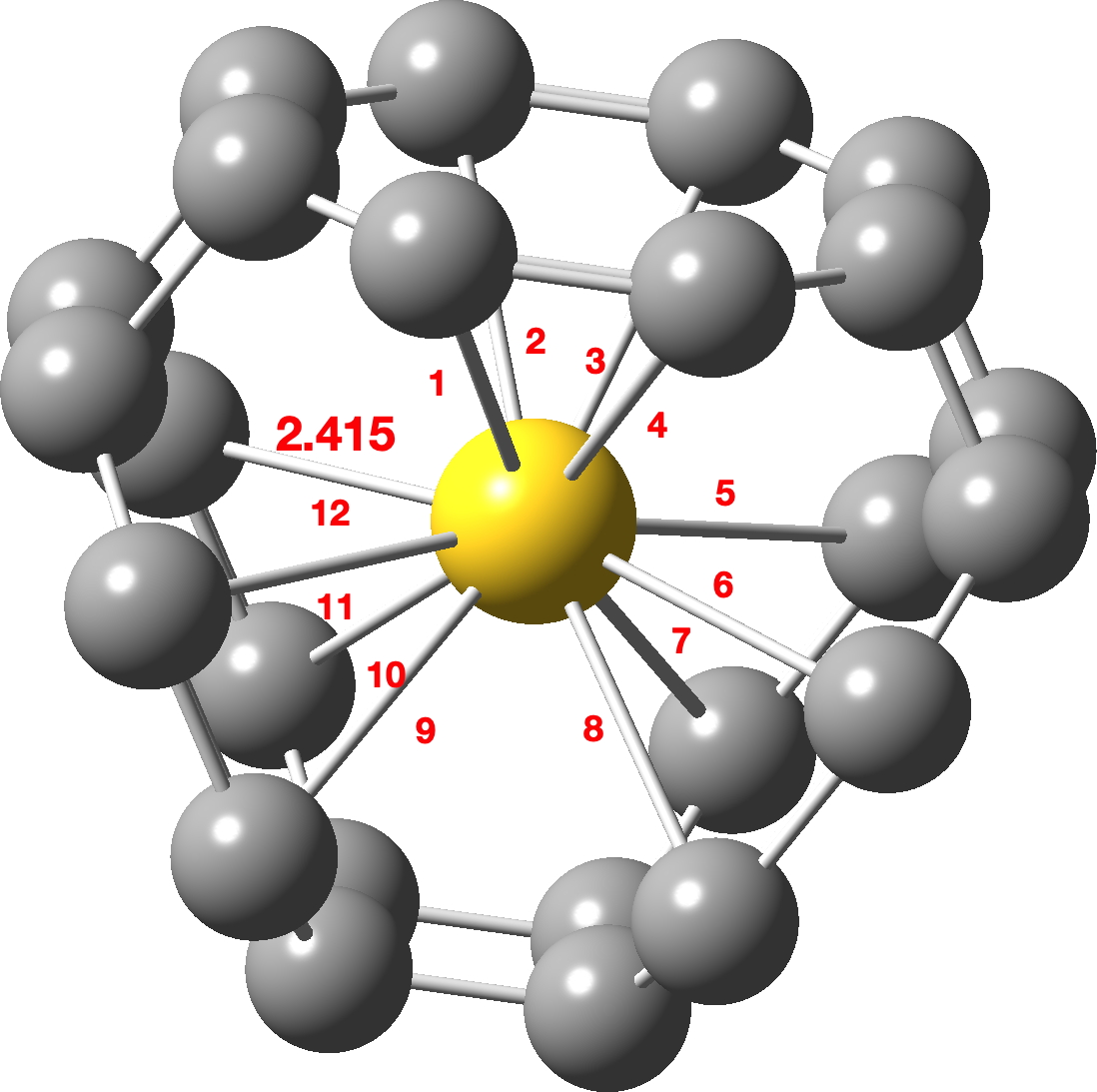
Ths simplest unit comprises three eight membered carbon rings, each connected by 4-membered rings to form a local structure with D3h symmetry and hence revealing twelve C-Au bonds of the same length; 2.415Å. Click on the image above to view a 3D model.
A larger section of the (polymeric) structure is shown below, now with D2h symmetry and again with twelve identical C-Au bond lengths
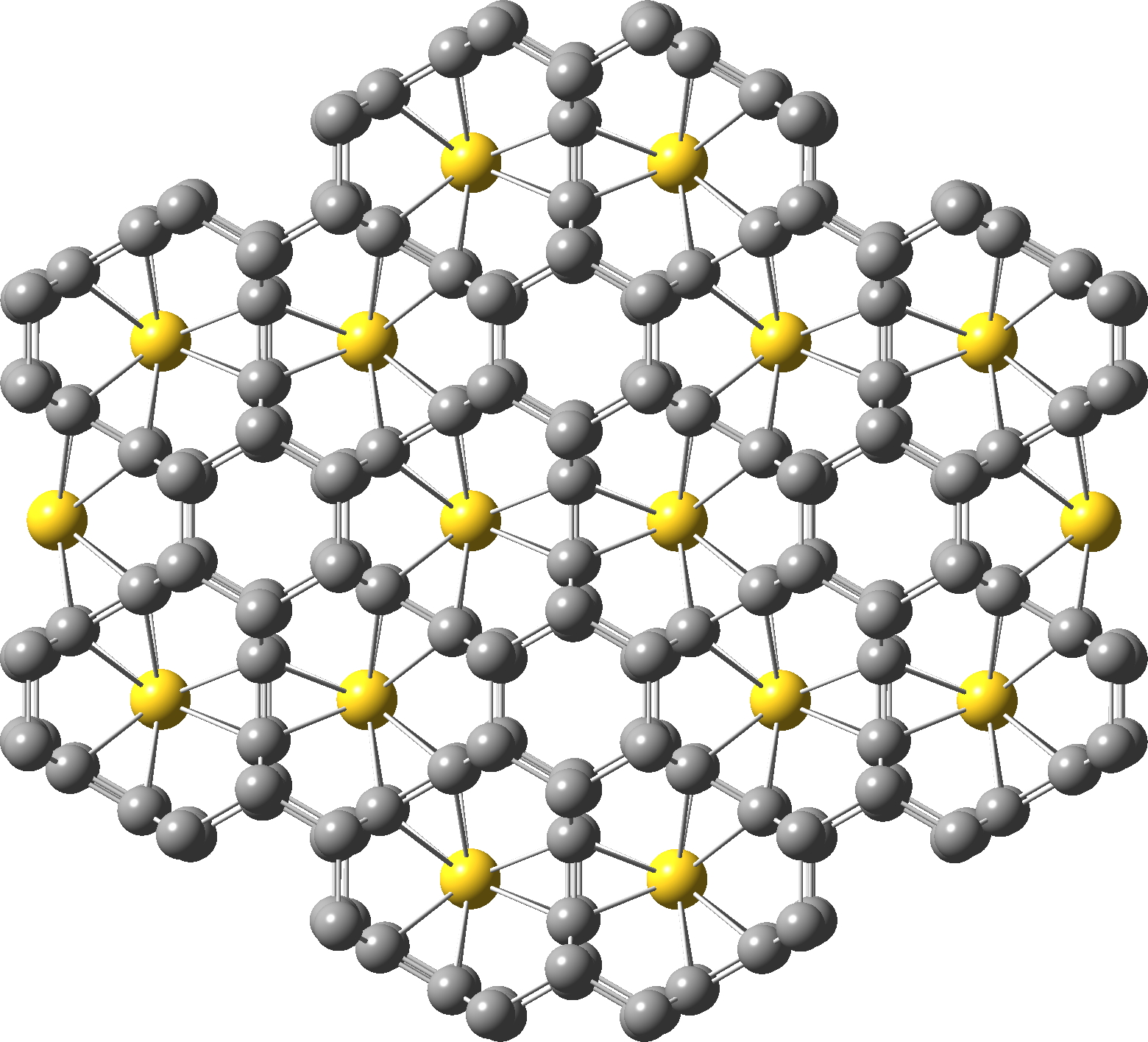
Is such coordination unusual?‡ Well, not for metal clusters, including Au clusters. There are in fact 2014 hits (1985 examples where Y is constrained to be a metal, hence 29 where the central atom is NOT a metal) in the Cambridge crystal structure database for the general search X12Y where X and Y can be any atom, with 244 for X=Au and 576 for X=O but none yet for X=C (the current example has not yet appeared in the distributed database). So certainly Au-pseudocarbyne is a unique and unusual molecule. This also shows that 3D coordinates can always be a useful adjunct to articles to allow quick access for spotting perhaps unexpected features with just a single click!
‡You might be surprised that a similar search finds 138 hits for X14Y and 16 for X16Y
References
- J. Wu, P. Tarakeshwar, S.G. Sayres, M. Meneghetti, H. Kim, J. Barreto, and P.R. Buseck, "Crystal structure of Au-pseudocarbyne(C6)", Scientific Reports, vol. 15, 2025. https://doi.org/10.1038/s41598-024-80359-5
- J. Wu, P. Tarakeshwar, S.G. Sayres, M. Meneghetti, H. Kim, J. Barreto, and P.R. Buseck, "Replication Data for: Crystal structure of Au-pseudocarbyne(C6)", 2024. https://doi.org/10.48349/asu/3twei0