HCl reacting with a carbonyl compound (say formaldehyde) sounds pretty simple. But often the simpler a thing looks, the more subtle it is under the skin. And this little reaction is actually my prelude to the next post.
The mechanism is studied using ωB97XD/6-311G(d,p) with a simulated solvent (acetic acid) included (but not explicit solvent setting up any hydrogen bonds).
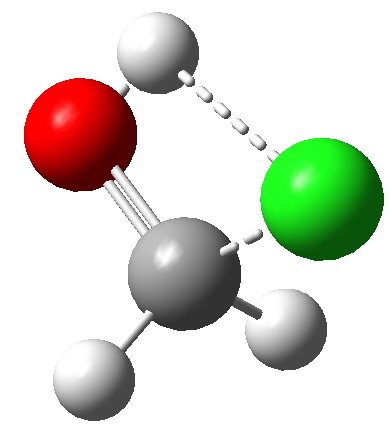 Transition state HCl + H2C=O. Click for 3D animation. |
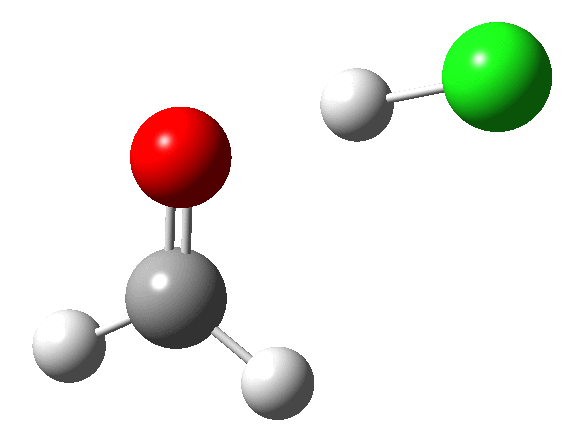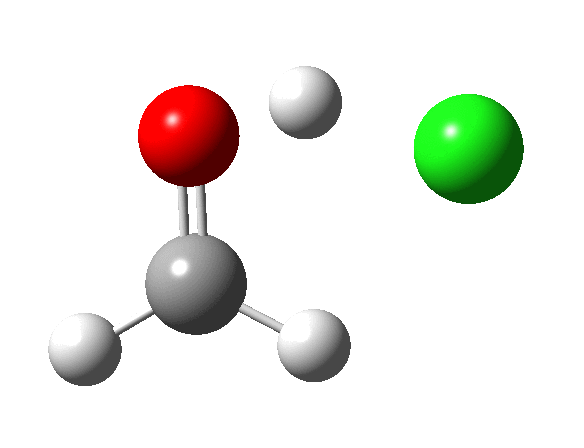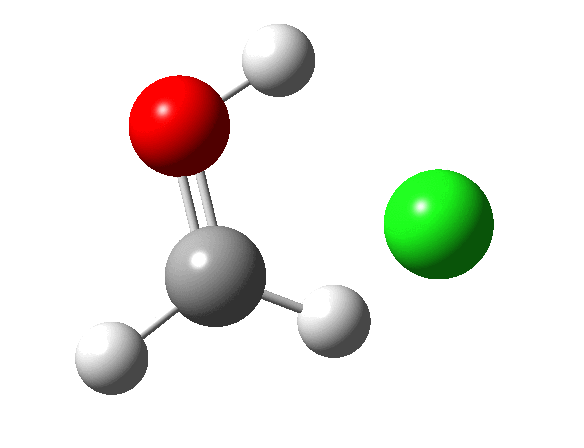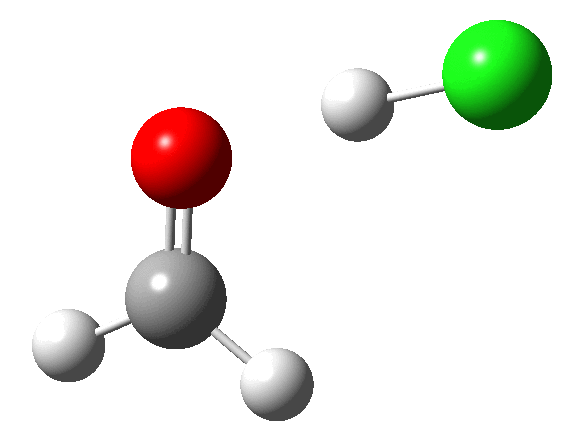 |
The transition state itself does not convey what is happening, largely because the transition state normal mode is mass-weighted. This leads to the heavier Cl not moving much, and the formaldehyde conducting a bee-dance like wag. For more detail, indeed insight, we need the intrinsic reaction coordinate (IRC):
- At IRC 4, the HCl starts by aligning itself into the plane of the formaldehyde, with the hydrogen targeting the in-plane lone pair on the carbonyl oxygen.
- At IRC 2.2, the hydrogen atom starts to transfer from the Cl to the O. As usual, a hydrogen transfer takes place very rapidly, and by IRC 1.7 the transfer is largely complete.
- At IRC 1.5, the chlorine, now shorn of its proton, starts to move out of the plane.
- At the transition state (IRC = 0.0) the chlorine is now inclined at an angle of about 45° with respect to the plane of the formaldehyde (which is still largely co-planar).
- Between IRC 0.0 and -2.0, the Cl…C bond starts to form, and the rotation goes to about 73°. It is held at this position because of an anomeric effect operating between one of the lone pairs on the oxygen atom, and the axis of the C-Cl σ* bond.
- The overall process is concerted, but quite asynchronous, in as much as the formation of the O…H bond distinctly precedes that of the C…Cl bond. These bonds form at a dihedral (torsional) angle of 73° with respect to each other and the need to align the two bonds in this manner means that they cannot form at the same rate!
Is this model a realistic one? Well, the missing component is hydrogen bonds. Between a solvent (this is being done by the way in acetic acid as simulated solvent) and the chlorine, which must assume a large measure of being actually a chloride anion, countered by the oxenium cation. It is possible that the reaction may actually therefore not be concerted, but it might stop at the half-way stage of an ion-pair before continuing its journey. The calculated barrier (~20 kcal/mol) is actually quite reasonable for a thermal reaction, but hydrogen-bond stabilisation might be expected to reduce this to what in effect would correspond to a very fast room-temperature reaction.
Well, HCl + H2C=O does not sound complicated. But you can trust this blog to take something simple and make it less so!
Tags: acetic acid, Tutorial material
[…] Henry Rzepa Chemistry with a twist « Surprises (?) in the addition of HCl to a carbonyl group. […]
Does it means that the real reaction could run in non-polar solvents? The reagents are gases and a little bit could be absorbed in hexane…