Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published[1]. That was then.
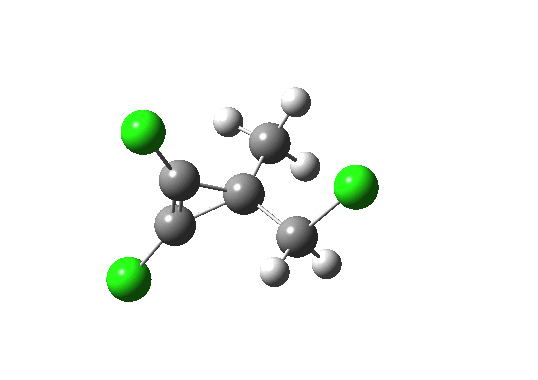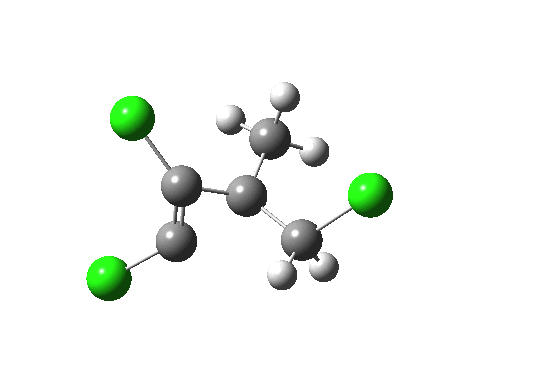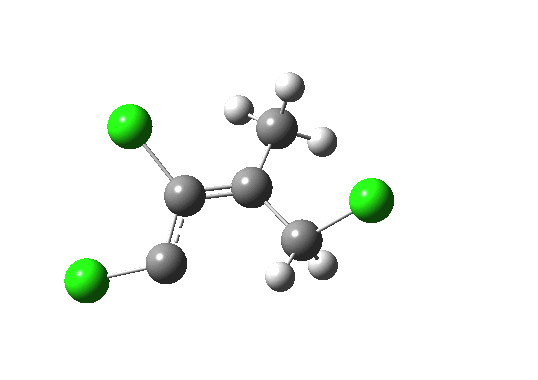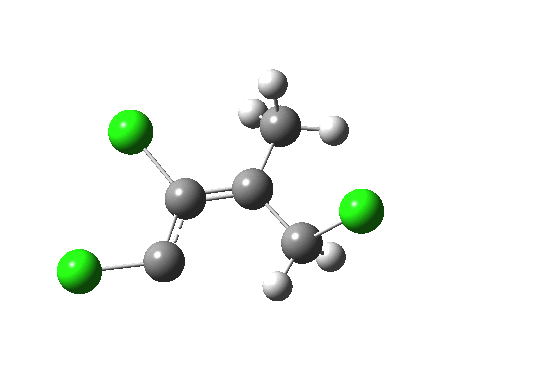
This is now. I have just finished a post on ring-opening reactions of oxirene, a 4n electron anti-aromatic ring. I was casting around for an example of a calculation done just before the modern Internet era, and happened upon the above. Although this was a mere 20 years ago, much of the detail had faded; I had not thought much about it in the intervening years, but I did recollect that although we had attributed the high stereoselectivity shown above to a stereoelectronic orbital alignment, I was not entirely happy with the interpretation. Put simply, we had relied on a molecular orbital diagram, and this diagram (in resplendent colour in the journal, one of the few being so published at that time, and for no colour charge to boot) was just too complicated. Arguably it was the fixated complexity (I remember at the time that it looked complicated no matter what the viewing angle was) that set me on the road to the use of the Web, and ultimately here to this blog. So I thought a re-analysis using modern algorithms and presentation might help simplify. The newly recalculated transition state (ωB97XD/6-311G(d,p)[2] looks like:
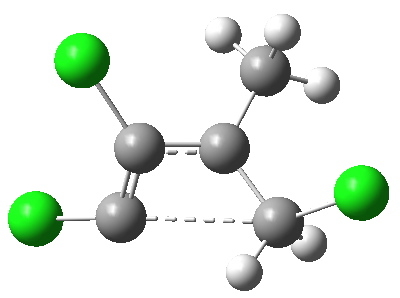
Transition state for ring opening of a cyclopropene. Click for 3D.
- The reaction is a 4n (n=1) electron electrocyclic ring opening and so according to the rules, should proceed with the formation/cleavage of an antarafacial bond. You might think that there are not quite enough substituents to reveal this stereochemistry, but there are if the carbene lone pair is included. So how to add the lone pair?
- Well, its coordinates can be computed using the ELF (electron localisation function). The relevant lone pair is ringed in red below. Using (old technology, i.e. a static figure) you may choose to believe me when I argue that this lone pair is above the plane of the forming ring from the perspective shown, whilst the terminus of the bond it forms is to the bottom. This defines an antarafacial component. Well, I might have carefully manipulated the viewing angle to show this. Now, in 2012 rather than 1992, you can load the 3D coordinates by clicking below, and check for yourself!
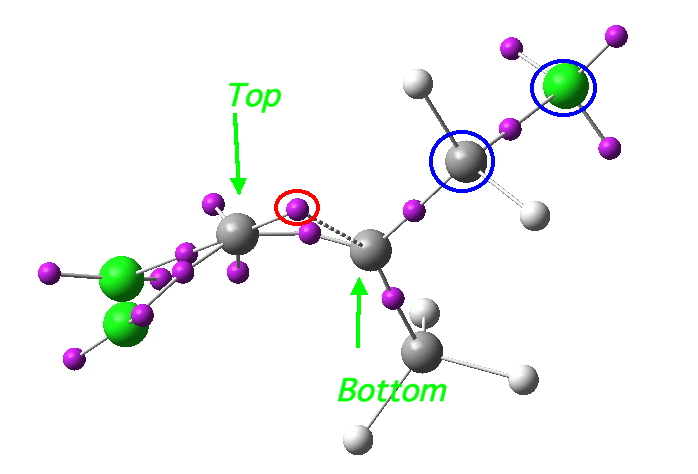
Lone pair centroid for the transition state. Click for 3D
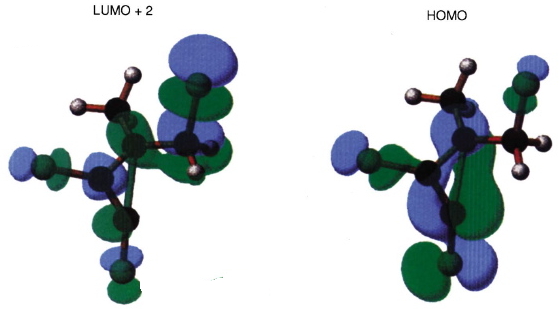
- What about the stereo-control? Take a look at the angle between the axis of the C-Cl bond (atoms ringed in blue) and the centroid of the carbene lone pair (red). It is about 162°, or almost anti-periplanar. A magic orientation in organic chemistry. Time to attack the orbitals again. Our published diagram looked as below. It shows the HOMO aligning with the LUMO+2 (if your eyes are not distracted by all the other detail).
But we can now simplify such a complex molecular orbital by using instead a localized version, an NBO. A little explanation is needed. The NBO orbital shown with red/blue phases is antibonding for the C-Cl bond. That with orange/purple is the carbene lone pair. Where orange overlaps with red, we have a positive overlap that stabilises the system. The NBO E2 perturbation energy is around 4.6 kcal/mol. Although this may seem small, it is actually quite large for a through-space interaction of this type. It is this stabilisation (amounting to ~ 1.6 kcal/mol in free energy) that accounts for the high selectivity for the stereoisomer shown above.
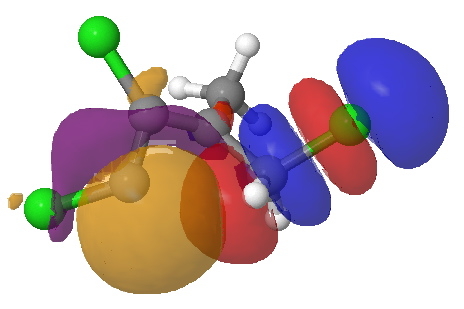
NBO for transition state. Click for 3D.
Well, I think that the passage of 20 years has enabled us to tidy up the origins of the stereoelectronic effect responsible for controlling this reaction, and to produce clearer diagrams which the reader can interactively explore for themselves. It did take 20 years to join things up though!
References
- M.S. Baird, J.R. Al Dulayymi, H.S. Rzepa, and V. Thoss, "An unusual example of stereoelectronic control in the ring opening of 3,3-disubstituted 1,2-dichlorocyclopropenes", Journal of the Chemical Society, Chemical Communications, pp. 1323, 1992. https://doi.org/10.1039/c39920001323
- H.S. Rzepa, "C 5 H 5 Cl 3", 2012. https://doi.org/10.14469/ch/14180
- H.S. Rzepa, "Gaussian Job Archive for C5H5Cl3", 2015. https://doi.org/10.6084/m9.figshare.1285422
Tags: Cambridge, chemist, conformational analysis, free energy, Historical, Internet era, London, pericyclic, perturbation energy, re-analysis using modern algorithms, Reaction Mechanism, Skolnik
[…] canines). This temporal warping can also be said to apply to computational chemistry. I previously revisited some computational work done in 1992, and here I rediscover another investigation from that year and that era. The aim in […]