2,3-Dibromofuran
2,3-Dibromofuran was prepared according to known procedures from furan-2-carboxylate via dibromination, hydrolysis of the
ester and decarboxylation [5].
The initial lithiation at this compound occurs at position 5 due to the electron-withdrawing effect of the neighbouring
oxygen atom. The fate of this primary metalated species and thus the product obtained after quenching with an electrophile
depends on the conditions applied during the reaction.
Halogen migration
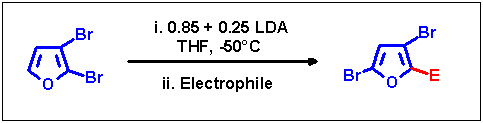
To induce migrations slow addition of externally prepared LDA to the starting compound was required. Trying out various
modifications led us to the following optimum conditions: a total of 1.1 equiv. of LDA was added in two portions
with a ratio of ca. 85:25 to the educt. After the first addition the reaction mixture was kept at -50 °C for 30 min.
During this time sufficient non-lithiated starting compound is available in the presence of metalated species allowing
the formation of 2,3,5-tribromofuran acting as a transbromination catalyst and thus the reactions leading to the rearranged
substitution pattern occur. Subsequently, the remaining LDA was added slowly over a period of 15 min to
complete the conversion, maintaining the reaction temperature constant for another 15 min. Quenching the resulting lithium
intermediate with various electrophiles afforded 2-substituted 3,5-dibromofurans in good yields.
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
78 |
| Cyclohexanone |
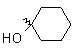 |
66 |
| Dimethylformamide |
-CHO |
70 |
| Iodomethane |
-CH3 |
84 |
| Dimethyl disulfide |
-SCH3 |
64 |
Prevention of halogen dance
As already outlined in the mechanism section, the key step for preventing halogen migrations
is to provide appropriate reaction conditions for a very rapid and complete initial lithiation step. In the case of
2,3-dibromofuran this goal was achieved by slowly adding the educt to 1.5 equiv. of LDA over a period of 30 min
at -80 °C. Reactions of the thus generated lithium intermediate yielded pure non-rearranged products only with
'rapid' electrophiles (chlorotrimethylsilane, cyclohexanone):
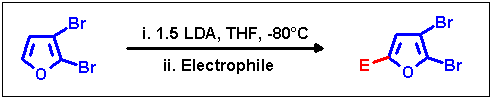
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
78 |
| Cyclohexanone |
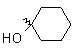 |
91 |
| Dimethylformamide |
-CHO |
64 |
While the reaction with dimethylformamide (which is a little less reactive) gave only a small amount of rearranged
by-product which was separable by recrystallization, quenching with 'slow' electrophiles such as iodomethane
resulted in inseparable mixtures of both isomers.