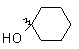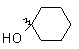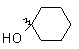2-Bromo-5-methylfuran
As there is no preparation for this compound described in literature, we had to develop a new route: bromination of 2-methylfuran
in DMF similar to the known preparation of 2,5-dibromofuran [6] turned out to be the most
effective solution [7].
In addition to the generally lower acidity of furan b-protons the electron-donating effect of the methyl group causes
the lithiation of this product to be even more difficult to achieve.
For both of the reaction types more rigorous lithiation conditions were therefore required.
Halogen migration
Under the usual lithiation conditions (LDA/THF) no metalation of 2-bromo-5-methylfuran was obtained, even on raising the
temperature to -20 °C. This failure prompted us to search for a more powerful metalating agent. The best results were
obtained with superbasic reagents of the LIDAKOR-type [8] (mixtures of alkyllithium compounds,
amines and potassium alcoholates) - the more frequently used LICKOR-reagents [9] (super-bases
from alkyllithium and alcoholates only) are not applicable in this case due to their ability for halogen metal exchange.
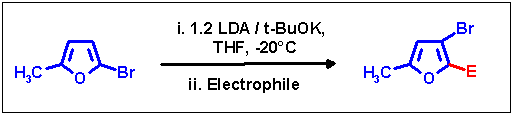
A superbasic reagent prepared from LDA and potassium tert-butanolate proved to be sufficient powerful for metalation
of the educt, and reaction conditions were developed which allowed the selective formation of the "rearranged" substitution
pattern: adding 2-bromo-5-methylfuran rapidly to freshly prepared LIDAKOR and subsequent quenching with various
electrophiles revealed 2-substituted 3-bromo-5-methylfurans free of HD-retained isomers in moderate yields.
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
44 |
| Cyclohexanone |
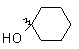 |
32 |
| Benzaldehyde |
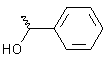 |
42 |
| Dimethylformamide |
-CHO |
30 |
| Iodomethane |
-CH3 |
29 |
| 1-Iodobutane |
-CH2CH2CH2CH3 |
28 |
| BrCN |
-Br |
31 |
In spite of performing thorough investigations and numerous experiments with various reaction times and temperatures,
it proved impossible to increase the yields. We believe the reason therefore to be mainly the weaker nucleophilicity
of the corresponding lithium intermediate as compared to the dibromo-lithium intermediates derived from the dibromofurans.
Prevention of halogen dance
As already discussed at the dibromofurans, slow addition of starting material to excess of metalation base proved to be
the method of choice to retain the substitution pattern. With 2-bromo-5-methylfuran as substrate neither LDA/THF
(no metalation at all) nor LIDAKOR/THF (halogen dance due to slow initial metalation) were applicable, changing the
solvent to THP was required - already successfully introduced for prevention reactions on 2,5-dibromofuran. Upon slow
addition of the substrate to 2.5 equiv. of LDA in THP at -50 °C no migration was observed, and after quenching
with various electrophiles and workup the corresponding 3-substituted 2-bromo-5-methylfurans were obtained without
HD isomers. As for the migration products reported above again moderate yields were obtained, which we were not able
to substantially increase by variation of reaction parameters.
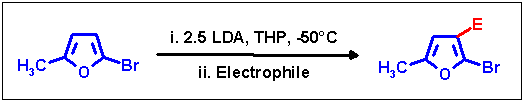
| Electrophile |
E |
Yield (%) |
| Chlorotrimethylsilane |
-Si(CH3)3 |
66 |
| Cyclohexanone |
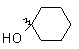 |
32 |
| Benzaldehyde |
 |
40 |
| Dimethylformamide |
-CHO |
26 |