

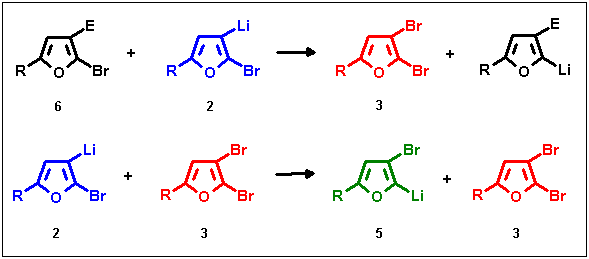
The sequence starts with lithiation of the starting compound 1 at the most acidic site (eqn. 1), followed by a key step common to all halogen migration reactions (eqn. 2): if the initial lithiation product 2 comes into contact with unreacted 1, a lithium-halogen exchange reaction leading to the dibrominated furan 3 takes place. A polybromo compound of this type is a prerequisite in all halogen dance examples known to us, serving as a co-catalyst for the two possible ensuing transmetalation steps (eqns. 3 and 4) which complete the migration. In eqn. 3 the transbrominating agent 3 is consumed under formation of scrambling product 5, but above all also under regeneration of starting compound 1, which according to eqn. 2 is responsible for 3 being formed again. In addition the initial lithiation product 2 and dibromide 3 produce the migration target 5 via an autocatalytic step as revealed by eqn. 4 - by these means to the advantage of a total rearrangement at least small amounts of 3 are sustained during the whole reaction time. Apparently reactive bromo-intermediates (and not bases) play the catalytic role - therefore we proposed the term aryl bromide catalysed dance (ABCD) reaction for such LDA-induced halogen migrations and prefer it over the term base-catalysed halogen dance (BCHD) reaction. Both processes represented by eqns. 3 and 4 give rise to the formation of the most stable, "final" lithium intermediate 5 with the Li-atom at the most acidic site.
Although the equilibrium positions of eqns. 2-4 do have some influence on the overall reaction rate, the main factor for a migration to occur is the simultaneous presence of 1 and 2 and their potential to react. These preconditions are mainly influenced by the sequence of addition and by the ratio of starting compounds:
Other factors that influence the reaction rate of the initial lithiation (eqn. 1) are the type of solvent (more polar solvents have been found to accelerate the metalation rate and are therefore helpful in preventing HD) and, to some extent, the temperature.
For this reason the successful completion of a prevention reaction is only possible using strong electrophiles (reacting with the primary lithiated species immediately) like aldehydes, ketones or chlorotrimethylsilane.