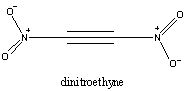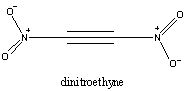Nitrocubanes
An overview:
As stated in the introduction, many HEDMs, especially those used as
explosives and propellants, contain much nitrogen and oxygen. Amongst the
most commonly found substituents on organic HEDMs is the nitro group (-NO2),
which combines both nitrogen and oxygen. These elements are useful in
explosives for the for the following reasons:
- Nitrogen gas (N2) has a very strong triple bond , and
the formation of this bond is a huge driving force for the reaction.
- Oxygen is used to oxidise the carbon in the organic framework
of most explosives to CO2. As the explosive is now no longer reliant
on gaseous oxygen to react, the availability of gas is no longer a limiting
factor for the speed of reaction. In effect, this allows for much faster
reacting explosives, and, with explosives, the speed of reaction is everything.
(In oxygen deficient explosives, oxygen rich solids, such as chlorates
(VII), can be added to aid the reaction, but these additions lower the
density of the explosive, decreasing its explosive power.)
- N2, CO2, and O2 (which will be produced
if there is excess oxygen beyond that which is needed for combustion of
the oxygen) are all gases, which increases the volume of the explosion products
compared to the explosive, and therefore increases its power.
- C-NO2 bonds and N-NO2 bonds are reasonably
shock-resistant, and therefore can be used in commercial explosives, unlike
other Nitrogen containing groups, such as -ONO2 and -N3,
which are not shock-resistant, and are highly dangerous.
Nitro-compounds are, in fact, the explosives of choice. So when researchers
started looking for cubane-derived HEDMs, the first port of call was the
nitrocubanes. As far as the researchers thought, the more nitro-groups
the better. And with that thought in mind they set out in search of the
Holy Grail - octanitrocubane (ONC), which was predicted to be one of the
most powerful explosives possible - it has a perfect oxygen balance (two
oxygens for each carbon), and was predicted to have a very high density,
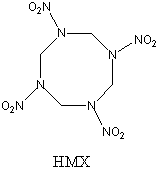
and to react very quickly, with a 1150-fold increase in volume1. This would make it 15-30% more powerful
than a very powerful and commonly-used explosive, HMX, and also better than
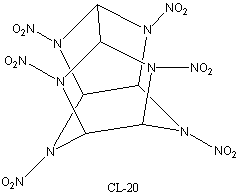
the best known usable explosive1, CL-20, which is often
said to be the most powerful non-nuclear explosive in existence3.
Tetranitrocubane
1,3,5,7-tetranitrocubane (C8H8(NO2)4) is the first nitrocubane that
will be looked at. Nitrocubane, although easy to make, is not nitrated enough
to be useful as an explosive, and neither are di- and tri-nitrocubanes,
as all of these compounds are more thermodynamically stable than cubane,
because the nitro-groups pull electron density out of the cubane system,
reducing bond strain. The first nitrocubane which has a higher enthalpy
of formation than the one with one less nitro-group is TNC, as it is when
there are 4 nitro-groups that the repulsive effects of having so many electronegative
groups so close together outweighs the increased stability of the cubane
system5. Regardless, 1,3,5,7-tetranitrocubane(TNC)
is worthy of mention as it was the first 'stumbling block' along the path
to ONC. TNC is synthesised from cubanecarboxylic acid chloride (cubylmethanoyl
chloride) as follows 3:
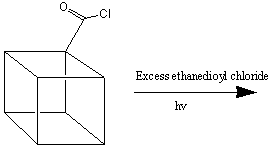
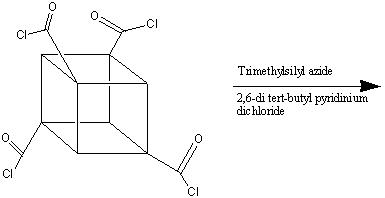
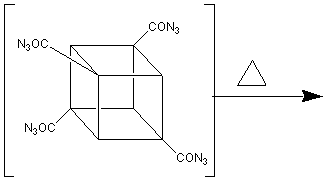
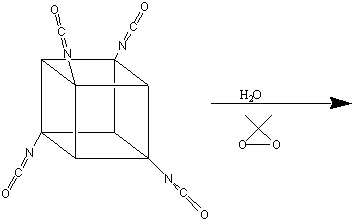
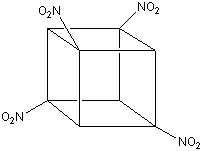
TNC
TNC is remarkably kinetically stable, just like cubane, surviving hammer
blows without reaction, which is quite extraordinary for an HEDM. It also
decomposes, rather than detonating, when melted. It is an extremely dense
(1.814g cm-3) crystalline solid, and as it is so dense, and
has a high enthalpy of formation, it would be expected to be a good explosive.
The detonation of TNC can be carried out, under controlled conditions,
and its explosive power exceeds predictions - unfortunately, these
results are, for the moment, being kept secret 1.
Pentanitrocubane and hexanitrocubane
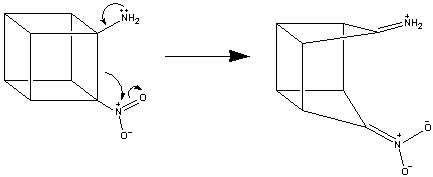
The above synthesis cannot generate cubanes with adjacent nitro groups.
This is because the fourth step of the synthesis (the reaction of polyisocyanatocubane
with dimethyldioxirane and water) necessitates, at some point, having an
amino group adjacent to a nitro group if any of the isocyanate groups
were next to each other. This can not be done, as a cubane structure which
has a strongly electron-withdrawing group next to a strongly electron-donating
group breaks up the cubane structure:
This means that to get more highly nitrated cubanes, TNC must be nitrated
directly. This was quite a challenge.
One of the most interesting things about the chemistry of TNC is its
acidity (pKa=21 1),
which is around 20 powers of 10 higher than that of cubane. This makes
it very easy to form salts of TNC, which is useful for synthesis - for example,
2,4,6,8-tetranitrocubyl sodium can be reacted with an alkyl halide to add
alkyl groups onto the cubane skeleton, which is a useful procedure. This
can also be done repeatedly, so that four new groups can be introduced.
Although this is useful, it does not lend itself to easy nitration - aliphatic
anions cannot normally be nitrated by nitro-cations (NO2+).
A new method of nitration needed to be developed before this could take place.
Luckily, a new method of nitration was developed - Interfacial Nitration.
This method, a solution of a salt containing the anion which
is to be nitrated is frozen to a glass. Then, solid N2O4
is deposited on the surface of the glass. When the glass is allowed to melt,
the anion is nitrated. This is a very odd reaction, and is very difficult
to explain - the same anion, mixed with solid N2O4
in the same solvent at just above the solvent's melting point will not react.
However, oddness aside, it gets good results.
This method allowed the synthesis of two more cubanes - 1,2,3 5,7-pentanitrocubane(PNC)
and 1,2,3,4,5,7-hexanitro-cubane(HNC). Both of these chemicals are stable,
crystalline solids, and very dense (PNC has a density of 1.96 gcm-3
at 21oC 3),
which makes them good candidates for explosives. PNC, like TNC, is stable
under ordinary conditions, and decomposes above 250oC 3 without detonation,
like most other cubanes. HNC is slightly more awkward - it is difficult to
isolate, as it cannot be purified by recrystallisation or solvent extraction,
owing to its instability in alcohols. It is therefore very difficult to say
exactly what its qualities are, as they could be affected by the solvent
(normally ethanenitrile, CH3CN). It also seems to be a typical cubane. Both
of these compounds are more acidic than TNC - PNC is 1000 times more acidic
than TNC, and HNC even more acidic than that 1. This is probably caused
by there being more highly electronegative nitro-groups pulling electron
density out of the cubane system, weakening the C-H bonds.
Heptanitrocubane
However, this method of nitration again proved insignificant to the task
- it could only result in a six-times nitrated cubane. Another method needed
to be thought of to add more nitro-groups.
The formation of heptanitrocubane (HpNC) can be achieved by adding four
equivalents of NaN(TMS)2 to TNC in a 1:1 THF:2-methyl THF
at -78oC. This is then then chilled to -125oC, and
excess N2O4 in cold 2-methylbutane added. The reaction
is allowed to proceed for one minute, before being quenched with nitric acid
in cold ethoxyethane. This is then added to water, and gives HpNC in high
yield. The whole series of transformations is shown below:1
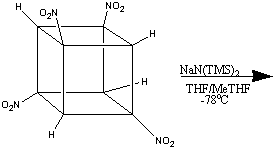
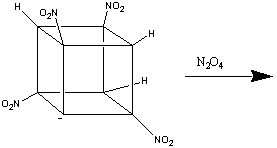
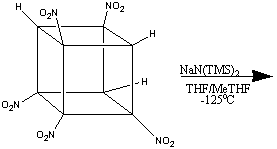
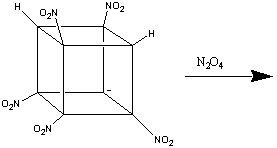
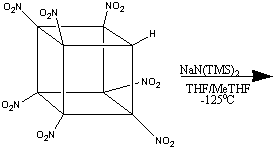
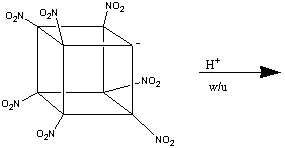
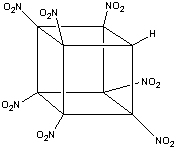
HpNC is a white, crystalline solid which is incredibly dense (2.028 g
cm-3 4) for a compound that consists
only of carbon, hydrogen, nitrogen, and oxygen. It decomposes, without detonation,
at above 200oC 4.
It is, generally, a fairly strong acid - it protonates liquid methanol to
give CH3OH2+. However, it is also sensitive
to base; NaF in methanol will catalyse its decomposition, and HpNC will decompose
violently, almost explosively, in the presence of pyridine.
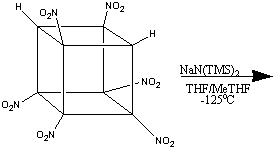
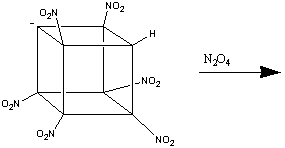
However, this method, unexplainably, proved unable to nitrate the 8th
carbon. Yet again, a new reaction had to be found before the goal of fully
nitrating cubane, and creating the 'perfect' cubane-derived HEDM was achieved.
The Holy Grail - Octanitrocubane
The eventual explanation for the non-formation of octanitrocubane was the
fact that the heptanitrocubyl anion is too stable to react with N2O4.
This led to the treatment of the heptanitrocubyl anion with the much stronger
oxidants, such as NOCl. The reaction of heptanitrocubyl lithium with excess
NOCl in CH2Cl2 at -78oC, followed by ozonation,
gives octanitrocubane in an approximately 50% yield1.
Octanitrocubane(ONC) is a white crystalline solid, which decomposes well
above 200oC, the temperature at which it sublimes, unchanged. It
also survives hammer taps, and seems to be able to be stored for long periods
- samples have survived unchanged for over a year. It has a density of 1.979g
cm-3 1,
which, although very high for a nitrated organic molecule, is still lower
than expected - indeed, it is lower than the density of HpNC! Even a simple
extrapolation of the densities of the other nitrocubanes would suggest that
ONC should have a density of around 2.06gcm-3 1. As density is extremely
important for the efficacy of an explosive, this could seem to be a bad sign
for ONC's explosive potential. However, nitro-compounds often have different
polymorphs (different crystal packing arrangements,) which have different
density - CL-20 has several polymorphs, with densities from 1.91 to 2.044g
cm-3 1.
There is no reason why ONC could not have other, more dense polymorphs. Polymorphs
are not easy to find - the only way to see if there are other polymorphs would
be to crystallise ONC from different solvents at different temperatures, and
see if the solids produced have different densities. This is very time-consuming.
However, predictions state that the most dense polymorph of ONC would have
a density of above 2.1g cm-3 1, which is enormous, and
would make it a very good explosive.
However, these concerns are almost irrelevant in the face of one huge obstacle
- the price. Cubane itself is extremely expensive. Explosives in mining are
often used in tons, and tens of kilograms are used in demolitions. The only
cubane derivative that is available in kilogram lots is the 1,5-dimethyl ester
of cubane, which costs around $60,000 per kilogram. Also, to get ONC, you
have to convert this ester into cubanecarboxylic acid, and then into TNC,
and then into HpNC, and then, finally, into ONC. All these steps make ONC
exorbitant, so to use it as an explosive when there are cheaper, almost-as-effective
alternatives, is extremely wasteful. Unless the price of cubane drops, cubane-based
explosives, and cubane-based HEDMs in general, will not be used commercially.
If the price of cubane does drop dramatically, then ONC is still unlikely
to be used, in favour of the much cheaper TNC, and/or the cheaper and denser
HpNC.
There is, however, one theory for the cheap production of ONC - four moles
of dinitroethyne are stoichiometrically the same as one mole of ONC. What's
more, theoretical calculations show that the reaction of four moles of dinitroethyne
to form one mole of ONC is has an enthalpy of around -400kJ mol-1
1. Of course, kinetics
may prevent this from occurring, and dinitroethyne has never been observed,
but if this reaction can occur, it might be a way to get ONC cheaply and easily.
Of course, this begs the question; if dinitroethyne is so high in energy,
why not use it as an HEDM?