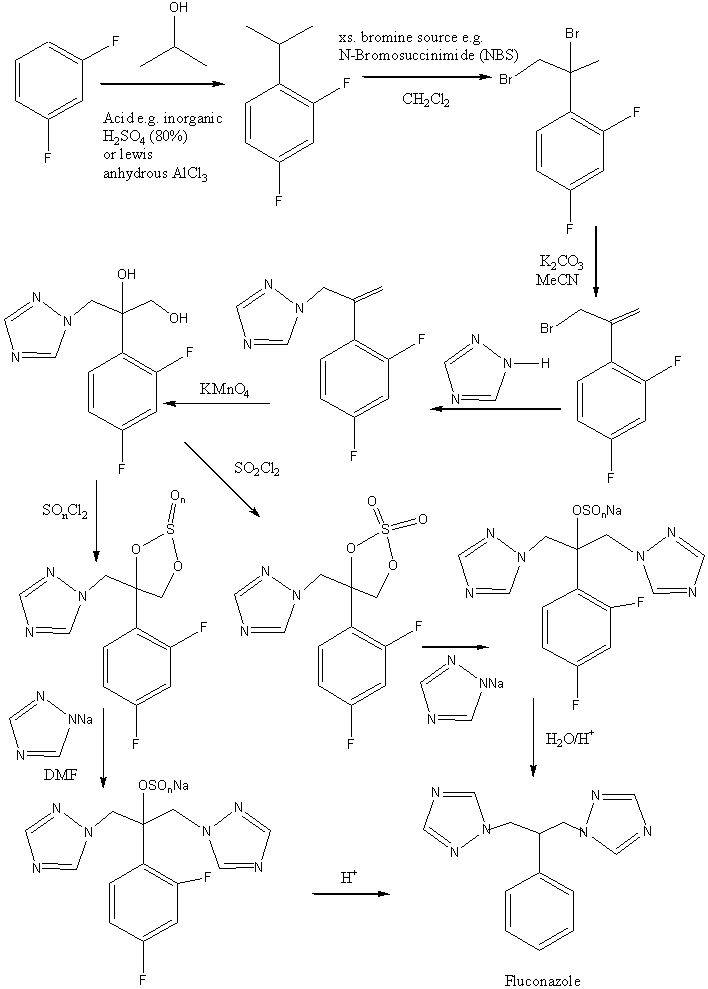
A commercial process is one in which the target product is made from readily available starting materials, easily handled, relatively inexpensive, and relatively safe to use resulting in a good yield.

The initial postualate for a commercial process involved preparing the cyclic sulphite directly from the diol at low temperature in the presence of a base but this only produced low yields of fluconazole. Yields of fluconazole remained low when altering reaction conditions due to competitive elimination processes abd bucleophilic attack on the sulphur atom.
To overcome these problems, a cyclic sulphate was ultilised as sulphates are more reactive than sulphites in displacement reactions. This minimised elimanaion reactions and furthermore the susceptability of the sulphur atom to nucleophilic attack was minimised as this would require the formation of a pentavalent intermediate in the transition state.
Using the metallic salt of 1,2,4-triazole increases the reaction rate since it has a greater affinity to participate in displacement reactions due to the completely polar N-Na bond. The yield of fluconazole from the diol is 55% as opposed to 42% when converting the epoxide to fluconazole.
1. Conversion an epoxide to Fluconazole
2. Lithiated or grignard difluorobenzene
to Fluconazole
3. Laboratory suitable method
4. Commercially viable method:
Sulphate process