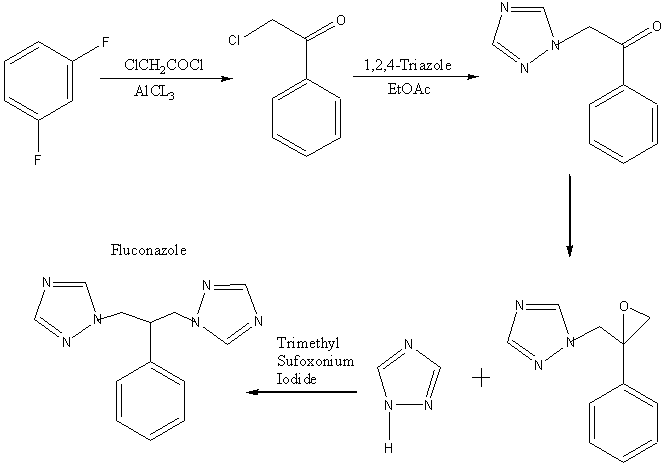

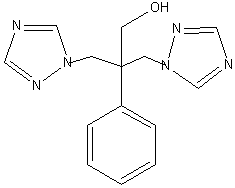
This method involves the conversion of the epoxide into fluconazole
which gives a good yield of arond 44%. The epoxide was prepared from
commercially available difluorobenzene which only took three apparently
simple steps and and was convienient due to its one-pot method however
this only results in a yield of about 4-8% of the epoxide. While
the ring opening of the epoxide is expected to take place at the least
hindered end, it is not impossible for the tertiary carbon to be attacked
and produce
1. Conversion an epoxide to Fluconazole
2. Lithiated or grignard difluorobenzene
to Fluconazole
3. Laboratory suitable method
4. Commercially viable method:
Sulphate process